Pharmacodynamics of cidofovir for vaccinia virus infection in an in vitro hollow-fiber infection model system
- PMID: 18852271
- PMCID: PMC2612146
- DOI: 10.1128/AAC.00708-08
Pharmacodynamics of cidofovir for vaccinia virus infection in an in vitro hollow-fiber infection model system
Abstract
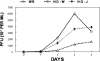
Variola major virus remains a potent weapon of bioterror. There is currently an investigational-new-drug application for cidofovir for the therapy of variola major virus infections. Stittelaar and colleagues compared the levels of effectiveness of postexposure smallpox vaccination (Elstree-RIVM) and antiviral treatment with cidofovir or an acyclic nucleoside phosphonate analogue 6-[2-(phosphonomethoxy)alkoxy]-2,4-diaminopyrimidine (HPMPO-DAPy) after lethal intratracheal infection of cynomolgus monkeys with monkeypox virus, a variola virus surrogate. Their results demonstrated that either compound was more effective than vaccination with the Ellstree vaccine (K. J. Stittelaar et al., Nature 439:745-748, 2006). An unanswered question is how to translate this information into therapy for poxvirus infections in people. In a proof-of-principle study, we used a novel in vitro hollow-fiber infection model system to determine the pharmacodynamics of vaccinia virus infection of HeLa-S3 cells treated with cidofovir. Our results demonstrate that the currently licensed dose of cidofovir of 5 mg/kg of body weight weekly with probenecid (which ameliorates nephrotoxicity) is unlikely to provide protection for patients intentionally exposed to Variola major virus. We further demonstrate that the antiviral effect is independent of the schedule of drug administration. Exposures (area under the concentration-time curve) to cidofovir that will have a robust protective effect will require doses that are 5 to 10 times that currently administered to humans. Such doses may cause nephrotoxicity, and therefore, approaches that include probenecid administration as well as schedules of administration that will help ameliorate the uptake of cidofovir into renal tubular epithelial cells need to be considered when addressing such treatment for people.
Figures





References
-
- Bray, M. 2003. Pathogenesis and potential antiviral therapy for complications of smallpox vaccination. Antivir. Res. 58:101-114. - PubMed
-
- Bray, M., M. Martinez, D. F. Smee, D. Kefauver, E. Thompson, and J. W. Huggins. 2000. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J. Infect. Dis. 181:10-19. - PubMed
-
- Breman, J. G., and I. Arita. 1980. The confirmation and maintenance of small pox eradication. N. Engl. J. Med. 303:1263-1273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

