Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo
- PMID: 18852290
- PMCID: PMC2571923
- DOI: 10.1084/jem.20080039
Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo
Abstract
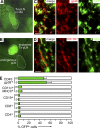
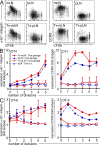
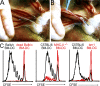
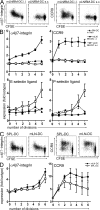
T cells primed in the gut-draining mesenteric lymph nodes (mLN) are imprinted to express alpha4beta7-integrin and chemokine receptor CCR9, thereby enabling lymphocytes to migrate to the small intestine. In vitro activation by intestinal dendritic cells (DC) or addition of retinoic acid (RA) is sufficient to instruct expression of these gut-homing molecules. We report that in vivo stroma cells, but not DC, allow the mLN to induce the generation of gut tropism. Peripheral LN (pLN) transplanted into the gut mesenteries fail to support the generation of gut-homing T cells, even though gut-derived DC enter the transplants and prime T cells. DC that fail to induce alpha4beta7-integrin and CCR9 in vitro readily induce these factors in vivo upon injection into mLN afferent lymphatics. Moreover, uniquely mesenteric but not pLN stroma cells express high levels of RA-producing enzymes and support induction of CCR9 on activated T cells in vitro. These results demonstrate a hitherto unrecognized contribution of stromal cell delivered signals, including RA, on the imprinting of tissue tropism in vivo.
Figures
References
-
- McDermott, M.R., and J. Bienenstock. 1979. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J. Immunol. 122:1892–1898. - PubMed
-
- Agace, W.W. 2006. Tissue-tropic effector T cells: generation and targeting opportunities. Nat. Rev. Immunol. 6:682–692. - PubMed
-
- Wagner, N., J. Lohler, E.J. Kunkel, K. Ley, E. Leung, G. Krissansen, K. Rajewsky, and W. Müller. 1996. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 382:366–370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

