Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes
- PMID: 18852301
- PMCID: PMC2568011
- DOI: 10.1083/jcb.200806137
Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes
Abstract
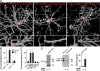
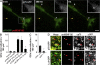
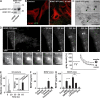
Activity-dependent secretion of brain-derived neurotrophic factor (BDNF) is thought to enhance synaptic plasticity, but the mechanisms controlling extracellular availability and clearance of secreted BDNF are poorly understood. We show that BDNF is secreted in its precursor form (pro-BDNF) and is then cleared from the extracellular space through rapid uptake by nearby astrocytes after theta-burst stimulation in layer II/III of cortical slices, a paradigm resulting in long-term potentiation of synaptic transmission. Internalization of pro-BDNF occurs via the formation of a complex with the pan-neurotrophin receptor p75 and subsequent clathrin-dependent endocytosis. Fluorescence-tagged pro-BDNF and real-time total internal reflection fluorescence microscopy in cultured astrocytes is used to monitor single endocytic vesicles in response to the neurotransmitter glutamate. We find that endocytosed pro-BDNF is routed into a fast recycling pathway for subsequent soluble NSF attachment protein receptor-dependent secretion. Thus, astrocytes contain an endocytic compartment competent for pro-BDNF recycling, suggesting a specialized form of bidirectional communication between neurons and glia.
Figures


References
-
- Aicardi, G., E. Argilli, S. Cappello, S. Santi, M. Riccio, H. Thoenen, and M. Canossa. 2004. Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. USA. 101:15788–15792. - PMC - PubMed
-
- Allen, N.J., and B.A. Barres. 2005. Signaling between glia and neurons: focus on synaptic plasticity. Curr. Opin. Neurobiol. 15:542–548. - PubMed
-
- Bezzi, P., V. Gundersen, J.L. Galbete, G. Seifert, C. Steinhauser, E. Pilati, and A. Volterra. 2004. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat. Neurosci. 7:613–620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

