Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions
- PMID: 18852364
- PMCID: PMC2756536
- DOI: 10.1161/CIRCULATIONAHA.108.793869
Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions
Abstract
Background: Two macrophage ABC transporters, ABCA1 and ABCG1, have a major role in promoting cholesterol efflux from macrophages. Peritoneal macrophages deficient in ABCA1, ABCG1, or both show enhanced expression of inflammatory and chemokine genes. This study was undertaken to elucidate the mechanisms and consequences of enhanced inflammatory gene expression in ABC transporter-deficient macrophages.
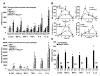
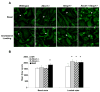
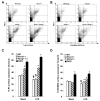
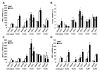
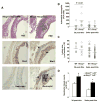
Methods and results: Basal and lipopolysaccharide-stimulated thioglycollate-elicited peritoneal macrophages showed increased inflammatory gene expression in the order Abca1(-/-)Abcg1(-/-)>Abcg1(-/-)>Abca1(-/-)>wild-type. The increased inflammatory gene expression was abolished in macrophages deficient in Toll-like receptor 4 (TLR4) or MyD88/TRIF. TLR4 cell surface concentration was increased in Abca1(-/-)Abcg1(-/-)>Abcg1(-/-)> Abca1(-/-)> wild-type macrophages. Treatment of transporter-deficient cells with cyclodextrin reduced and cholesterol-cyclodextrin loading increased inflammatory gene expression. Abca1(-/-)Abcg1(-) bone marrow-derived macrophages showed enhanced inflammatory gene responses to TLR2, TLR3, and TLR4 ligands. To assess in vivo relevance, we injected intraperitoneally thioglycollate in Abcg1(-/-) bone marrow-transplanted, Western diet-fed, Ldlr-deficient mice. This resulted in a profound inflammatory infiltrate in the adventitia and necrotic core region of atherosclerotic lesions, consisting primarily of neutrophils.
Conclusions: The results suggest that high-density lipoprotein and apolipoprotein A-1 exert anti-inflammatory effects by promoting cholesterol efflux via ABCG1 and ABCA1 with consequent attenuation of signaling via Toll-like receptors. In response to a peripheral inflammatory stimulus, atherosclerotic lesions containing Abcg1(-/-) macrophages experience an inflammatory "echo," suggesting a possible mechanism of plaque destabilization in subjects with low high-density lipoprotein levels.
Conflict of interest statement
Figures


References
-
- Gordon DJ, Rifkind BM. High-density lipoprotein-the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. - PubMed
-
- Okamoto H, Yonemori F, Wakitani K, Minowa T, Maeda K, Shinkai H. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature. 2000;406:203–207. - PubMed
-
- Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58:342–374. - PubMed
-
- Oram JF, Lawn RM, Garvin MR, Wade DP. ABCA1 is the cAMP- inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J Biol Chem. 2000;275:34508–34511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

