Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis
- PMID: 18852366
- PMCID: PMC2729441
- DOI: 10.1161/CIRCULATIONAHA.108.785881
Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis
Abstract
Background: To enable intravascular detection of inflammation in atherosclerosis, we developed a near-infrared fluorescence (NIRF) catheter-based strategy to sense cysteine protease activity during vascular catheterization.
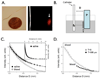
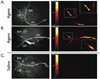
Methods and results: The NIRF catheter design was based on a clinical coronary artery guidewire. In phantom studies of NIRF plaques, blood produced only a mild (<30%) attenuation of the fluorescence signal compared with saline, affirming the favorable optical properties of the NIR window. Catheter evaluation in vivo used atherosclerotic rabbits (n=11). Rabbits received an injection of a cysteine protease-activatable NIRF imaging agent (Prosense750; excitation/emission, 750/770 nm) or saline. Catheter pullbacks through the blood-filled iliac artery detected NIRF signals 24 hours after injection of the probe. In the protease agent group, the in vivo peak plaque target-to-
Background: <0.05). Ex vivo fluorescence reflectance imaging corroborated these results (target-to-
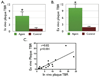
Background: <0.01). In the protease group only, saline flush-modulated NIRF signal profiles further distinguished atheromata from normal segments in vivo (P<0.01). Good correlation between the in vivo and ex vivo plaque target-to-
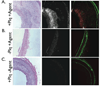
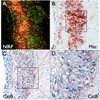
Background: =0.82, P<0.01). Histopathological analyses demonstrated strong NIRF signal in plaques only from the protease agent group. NIRF signals colocalized with immunoreactive macrophages and the cysteine protease cathepsin B.
Conclusions: An intravascular fluorescence catheter can detect cysteine protease activity in vessels the size of human coronary arteries in real time with an activatable NIRF agent. This strategy could aid in the detection of inflammation and high-risk plaques in small arteries.
Figures



References
-
- Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. - PubMed
-
- Jaffer FA, Libby P, Weissleder R. Molecular imaging of cardiovascular disease. Circulation. 2007;116:1052–1061. - PubMed
-
- Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. - PubMed
-
- Chen J, Tung CH, Mahmood U, Ntziachristos V, Gyurko R, Fishman MC, Huang PL, Weissleder R. In vivo imaging of proteolytic activity in atherosclerosis. Circulation. 2002;105:2766–2771. - PubMed
-
- Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, Weissleder R, Libby P. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006;114:55–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

