Nicotine decreases DNA methyltransferase 1 expression and glutamic acid decarboxylase 67 promoter methylation in GABAergic interneurons
- PMID: 18852456
- PMCID: PMC2570996
- DOI: 10.1073/pnas.0808699105
Nicotine decreases DNA methyltransferase 1 expression and glutamic acid decarboxylase 67 promoter methylation in GABAergic interneurons
Abstract
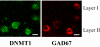
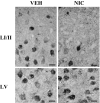
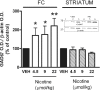
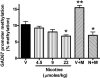
Tobacco smoking is frequently abused by schizophrenia patients (SZP). The major synaptically active component inhaled from cigarettes is nicotine, hence the smoking habit of SZP may represent an attempt to use nicotine self-medication to correct (i) a central nervous system nicotinic acetylcholine receptor (nAChR) dysfunction, (ii) DNA-methyltransferase 1 (DMT1) overexpression in GABAergic neurons, and (iii) the down-regulation of reelin and GAD(67) expression caused by the increase of DNMT1-mediated hypermethylation of promoters in GABAergic interneurons of the telencephalon. Nicotine (4.5-22 micromol/kg s.c., 4 injections during the 12-h light cycle for 4 days) decreases DNMT1 mRNA and protein and increases GAD(67) expression in the mouse frontal cortex (FC). This nicotine-induced decrease of DNMT1 mRNA expression is greater (80%) in laser microdissected FC layer I GABAergic neurons than in the whole FC (40%), suggesting selectivity differences for the specific nicotinic receptor populations expressed in GABAergic neurons of different cortical layers. The down-regulation of DNMT1 expression induced by nicotine in the FC is also observed in the hippocampus but not in striatal GABAergic neurons. Furthermore, these data show that in the FC, the same doses of nicotine that decrease DNMT1 expression also (i) diminished the level of cytosine-5-methylation in the GAD(67) promoter and (ii) prevented the methionine-induced hypermethylation of the same promoter. Pretreatment with mecamylamine (6 micromol/kg s.c.), an nAChR blocker that penetrates the blood-brain barrier, prevents the nicotine-induced decrease of FC DNMT1 expression. Taken together, these results suggest that nicotine, by activating nAChRs located on cortical or hippocampal GABAergic interneurons, can up-regulate GAD(67) expression via an epigenetic mechanism. Nicotine is not effective in striatal medium spiny GABAergic neurons that primarily express muscarinic receptors.
Conflict of interest statement
Conflict of interest statement: M.H. is associated with Pfizer Global Research and Development.
Figures

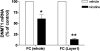
References
-
- Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: Potential new candidates for the treatment of schizophrenia. Psychopharmacology. 2004;174:54–56. - PubMed
-
- Leonard S, et al. Smoking and schizophrenia: Abnormal nicotinic receptor expression. Eur J Pharmacol. 2000;393:237–242. - PubMed
-
- Breese CR, et al. Abnormal regulation of high-affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

