A Schiff base connectivity switch in sensory rhodopsin signaling
- PMID: 18852467
- PMCID: PMC2571000
- DOI: 10.1073/pnas.0807486105
A Schiff base connectivity switch in sensory rhodopsin signaling
Abstract
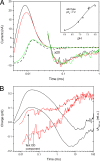
Sensory rhodopsin I (SRI) in Halobacterium salinarum acts as a receptor for single-quantum attractant and two-quantum repellent phototaxis, transmitting light stimuli via its bound transducer HtrI. Signal-inverting mutations in the SRI-HtrI complex reverse the single-quantum response from attractant to repellent. Fast intramolecular charge movements reported here reveal that the unphotolyzed SRI-HtrI complex exists in two conformational states, which differ by their connection of the retinylidene Schiff base in the SRI photoactive site to inner or outer half-channels. In single-quantum photochemical reactions, the conformer with the Schiff base connected to the cytoplasmic (CP) half-channel generates an attractant signal, whereas the conformer with the Schiff base connected to the extracellular (EC) half-channel generates a repellent signal. In the wild-type complex the conformer equilibrium is poised strongly in favor of that with CP-accessible Schiff base. Signal-inverting mutations shift the equilibrium in favor of the EC-accessible Schiff base form, and suppressor mutations shift the equilibrium back toward the CP-accessible Schiff base form, restoring the wild-type phenotype. Our data show that the sign of the behavioral response directly correlates with the state of the connectivity switch, not with the direction of proton movements or changes in acceptor pK(a). These findings identify a shared fundamental process in the mechanisms of transport and signaling by the rhodopsin family. Furthermore, the effects of mutations in the HtrI subunit of the complex on SRI Schiff base connectivity indicate that the two proteins are tightly coupled to form a single unit that undergoes a concerted conformational transition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Ruiz-Gonzalez MX, Marin I. New insights into the evolutionary history of type 1 rhodopsins. J Mol Evol. 2004;58:348–358. - PubMed
-
- Spudich JL, Jung KH. In: Handbook of Photosensory Receptors. Briggs WR, Spudich JL, editors. Weinheim, Germany: Wiley VCH; 2005. pp. 1–21.
-
- Sharma AK, Spudich JL, Doolittle WF. Microbial rhodopsins: Functional versatility and genetic mobility. Trends Microbiol. 2006;14:463–469. - PubMed
-
- Spudich JL. The multitalented microbial sensory rhodopsins. Trends Microbiol. 2006;14:480–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

