Network model of survival signaling in large granular lymphocyte leukemia
- PMID: 18852469
- PMCID: PMC2571012
- DOI: 10.1073/pnas.0806447105
Network model of survival signaling in large granular lymphocyte leukemia
Abstract
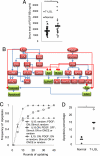
T cell large granular lymphocyte (T-LGL) leukemia features a clonal expansion of antigen-primed, competent, cytotoxic T lymphocytes (CTL). To systematically understand signaling components that determine the survival of CTL in T-LGL leukemia, we constructed a T-LGL survival signaling network by integrating the signaling pathways involved in normal CTL activation and the known deregulations of survival signaling in leukemic T-LGL. This network was subsequently translated into a predictive, discrete, dynamic model. Our model suggests that the persistence of IL-15 and PDGF is sufficient to reproduce all known deregulations in leukemic T-LGL. This finding leads to the following predictions: (i) Inhibiting PDGF signaling induces apoptosis in leukemic T-LGL. (ii) Sphingosine kinase 1 and NFkappaB are essential for the long-term survival of CTL in T-LGL leukemia. (iii) NFkappaB functions downstream of PI3K and prevents apoptosis through maintaining the expression of myeloid cell leukemia sequence 1. (iv) T box expressed in T cells (T-bet) should be constitutively activated concurrently with NFkappaB activation to reproduce the leukemic T-LGL phenotype. We validated these predictions experimentally. Our study provides a model describing the signaling network involved in maintaining the long-term survival of competent CTL in humans. The model will be useful in identifying potential therapeutic targets for T-LGL leukemia and generating long-term competent CTL necessary for tumor and cancer vaccine development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



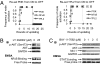
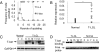
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

