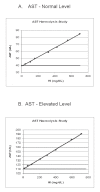
Interference testing
Abstract
* Interference occurs when a substance or process falsely alters an assay result. * Interferences are classified as endogenous or exogenous. Endogenous interference originates from substances present in the patient's own specimen. Exogenous interferences are substances introduced into the patient's specimen. * To perform interference studies, proper planning is required. * Interference from haemolysis, icterus and lipaemia are most frequently studied. Haemolysis affects more analytes than does any other type of interference. * Protein interferences are most often associated with paraproteins and predominantly with IgM or IgG and rarely with IgA. * Drug interference may be due to the parent drug, metabolite(s) or additives in the drug preparation. * Collection tube components can affect determination of analytes. * Carryover interference typically occurs when analyte from a high concentration sample (or reagent) is incompletely removed by the analytical system's washing process, whether probe, mixer or cuvette washing. * Immunoassay interferences are most commonly due to antibodies (generally polyclonal). They may be autoantibodies (e.g. in thyroid disease) or heterophile antibodies that predominantly interfere in two-site immunometric (sandwich) assays, forming a bridge between capture and detection antibodies. * Determining if interference is significant requires deviation limits from the original result. * Once interferences are identified during method evaluation or in general use, there is a need to establish procedures for handling affected results as part of the quality system.
Figures
References
-
- Dimeski G, Clague AE. Bicarbonate interference with chloride ion selective electrodes. Clin Chem. 2004;50:1106–7. - PubMed
-
- Clinical and Laboratory Standards Institute. Interference Testing in Clinical Chemistry; approved guideline. Wayne, PA, USA: CLSI; 2005. CLSI document EP7-P.
-
- Young’s Effects Online. [(Accessed 21 May 2008)]. https://www.fxol.org/aaccweb/
-
- Young DS. Effects of drugs on clinical laboratory tests. 5. Vol. 2. Washington DC, USA: AACC Press; 2000.
-
- Meites S. Reproducibly simulating hemolysis, for evaluating its interference with chemical methods. Clin Chem. 1973;19:1319. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous