North American neonatal extracorporeal membrane oxygenation (ECMO) devices and team roles: 2008 survey results of Extracorporeal Life Support Organization (ELSO) centers
- PMID: 18853828
- PMCID: PMC4680642
North American neonatal extracorporeal membrane oxygenation (ECMO) devices and team roles: 2008 survey results of Extracorporeal Life Support Organization (ELSO) centers
Abstract
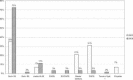
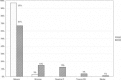
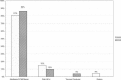
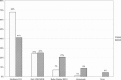
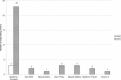
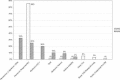
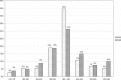
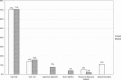
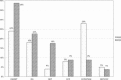
In early 2008, surveys of active extracorporeal membrane oxygenation (ECMO) centers in North America were conducted by electronic mail regarding neonatal ECMO equipment and professional staff. Eighty of 103 (78%) North American ECMO centers listed in the Extracorporeal Life Support Organization directory as neonatal centers responded to the survey. Of the responding centers, 82.5% routinely used roller pumps for neonatal ECMO, and the remaining 17.5% used centrifugal pumps. Silicone membrane oxygenators were used by 67% of the respondents, whereas 19% used micro-porous hollow fiber oxygenators, and 14% used polymethylpentene hollow fiber oxygenators. Of the silicone membrane oxygenator users, 86% used the Medtronic Ecmotherm heat exchanger, 10% used the Gish HE-4 heat exchanger, and 4% used the Terumo Conducer device. Sixty-four percent of the responding centers used some form of in-line blood gas monitoring. Six percent of the centers used a bubble trap in the arterial line, and 5% used an arterial line filter. A bladder was used by 85% of the centers, and 4% of these used a mechanical bladder box for servo regulation; the remaining 96% used pressure servo regulation. An air bubble detector was used by 88% of the responding centers. A surface coating was used by 44% of the centers on all their neonatal ECMO patients. Thirty-one percent of the centers use an activated clotting time of 180-220 seconds. At 54% of the responding centers, perfusionists were involved with the ECMO program, registered nurses were involved at 70% of the centers, and respiratory therapists were involved at 46% of the centers. Compared with a 2002 survey, silicone membrane use is declining, and the use of centrifugal blood pumps and coated ECMO circuits is becoming more apparent. ECMO teams are still multidisciplinary, made up of combinations of registered nurses, respiratory therapists, and perfusionists.
Figures
References
-
- Hill J, Deleval M, Fallat R, et al. Acute respiratory insufficiency. Treatment with prolonged extracorporeal oxygenation. J Thorac Cardiovasc Surg. 1972;64:551–62. - PubMed
-
- ECMO Registry. Report of the Extracorporeal Life Support Organization. Ann Arbor, MI: Extracorporeal Life Support Organization; 2008.
-
- Lawson DS, Walczak R, Lawson AF, et al. North American extra-corporeal membrane devices: 2002 survey results. J Extra Corpor Technol. 2004;36:16–21. - PubMed
-
- Bahrami KR, Van Meurs KP.. ECMO for neonatal respiratory failure. Semin Perinatol. 2005;29:15–23. - PubMed
-
- ECMO Registry. Report of the Extracorporeal Life Support Organization. Ann Arbor, MI: Extracorporeal Life Support Organization; 2002.
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
