Zolpidem extended-release 12.5 mg associated with improvements in work performance in a 6-month randomized, placebo-controlled trial
- PMID: 18853934
- PMCID: PMC2572742
Zolpidem extended-release 12.5 mg associated with improvements in work performance in a 6-month randomized, placebo-controlled trial
Abstract
Background: Although most research on pharmacotherapy for chronic insomnia focuses on changes in sleep outcomes, the functional impact of treatment is also of great importance to patients, families, physicians, and employers.
Objective: To analyze changes in functioning at work (or work performance) among a subset of employed subjects (N = 752) from a 24-week, randomized, double-blind, placebo-controlled trial of zolpidem extended-release 12.5 mg taken nightly, at least 3 nights per week, by healthy adults with chronic insomnia.
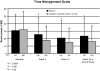
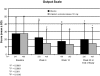
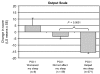
Methods: Using 2 scales (Time Management and Work Output) from the Work Limitations Questionnaire (WLQ), subjects' health-related work limitations were evaluated at baseline, week 4, week 12, and week 24 (end of study) or premature discontinuation. To compare zolpidem extended-release 12.5 mg with placebo, within-group and between-group differences were analyzed and effect sizes were computed. The relationship of WLQ scores to scores on the Patient Global Impression, Item 1 (PGI-1), scale, the primary outcome measure for benefit to sleep, was also analyzed. Data were obtained from August 31, 2004 through January 6, 2006.
Results: Scores on both WLQ scales were substantially elevated at baseline in this population, reflecting impairment relative to healthy controls. The zolpidem extended-release 12.5 mg group had significantly greater improvement at all time points on the WLQ Time Management (P < 0.0001) and Work Output (P < 0.01) scales. Effect size analysis confirmed the clinical relevance of these improvements. Subjects rating their sleep as improved on the PGI-1 had significantly greater improvement on both WLQ scales at week 12 than did those who reported no benefit or worsening (P < 0.01).
Conclusions: Employed adults with chronic insomnia treated with zolpidem extended-release 12.5 mg experienced significantly improved work performance over 24 weeks.
Figures

Similar articles
-
Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial.J Clin Psychiatry. 2011 Jul;72(7):914-28. doi: 10.4088/JCP.09m05571gry. Epub 2010 Dec 28. J Clin Psychiatry. 2011. PMID: 21208597 Clinical Trial.
-
Efficacy and safety of zolpidem extended release in elderly primary insomnia patients.Am J Geriatr Psychiatry. 2008 Jan;16(1):44-57. doi: 10.1097/JGP.0b013e3181256b01. Am J Geriatr Psychiatry. 2008. PMID: 18165461 Clinical Trial.
-
Efficacy and safety of zolpidem-MR: a double-blind, placebo-controlled study in adults with primary insomnia.Sleep Med. 2006 Aug;7(5):397-406. doi: 10.1016/j.sleep.2006.04.008. Epub 2006 Jul 3. Sleep Med. 2006. PMID: 16815744 Clinical Trial.
-
Zolpidem extended-release: a single insomnia treatment option for sleep induction and sleep maintenance symptoms.Am J Ther. 2007 May-Jun;14(3):299-305. doi: 10.1097/MJT.0b013e31804c7292. Am J Ther. 2007. PMID: 17515707 Review.
-
Treatment options for insomnia--pharmacodynamics of zolpidem extended-release to benefit next-day performance.Postgrad Med. 2008 Sep;120(3):161-71. doi: 10.3810/pgm.2008.09.1916. Postgrad Med. 2008. PMID: 18824834 Review.
Cited by
-
Confirmatory factor analysis of the Work Limitations Questionnaire (WLQ-25) in Workers' Compensation Claimants with chronic upper-limb disorders.J Occup Rehabil. 2013 Jun;23(2):228-38. doi: 10.1007/s10926-012-9397-6. J Occup Rehabil. 2013. PMID: 23117847
-
Insomnia and the performance of US workers: results from the America insomnia survey.Sleep. 2011 Sep 1;34(9):1161-71. doi: 10.5665/SLEEP.1230. Sleep. 2011. PMID: 21886353 Free PMC article.
-
The Comparative Effectiveness and Safety of Insomnia Drugs: A Systematic Review and Network Meta-Analysis of 153 Randomized Trials.Drugs. 2023 May;83(7):587-619. doi: 10.1007/s40265-023-01859-8. Epub 2023 Mar 22. Drugs. 2023. PMID: 36947394
-
Management of insomnia: update and new approaches.Nat Sci Sleep. 2010 Jul 28;2:127-38. doi: 10.2147/nss.s6642. Print 2010. Nat Sci Sleep. 2010. PMID: 23616705 Free PMC article.
-
Posttraumatic stress disorder symptoms and sleep in the daily lives of World Trade Center responders.J Occup Health Psychol. 2019 Dec;24(6):689-702. doi: 10.1037/ocp0000158. Epub 2019 Jun 17. J Occup Health Psychol. 2019. PMID: 31204820 Free PMC article.
References
-
- Krystal AD. Treating the health, quality of life, and functional impairments in insomnia. J Clin Sleep Med. 2007;3:63–72. - PubMed
-
- Leger D, Guilleminault C, Bader G, et al. Medical and socio-professional impact of insomnia. Sleep. 2002;25:625–9. - PubMed
-
- Leger D, Massuel MA, Metlaine A. Professional correlates of insomnia. Sleep. 2006;29:171–8. - PubMed
-
- Zammit GK, Weiner J, Damato N, et al. Quality of life in people with insomnia. Sleep. 1999;22(suppl 2):S379–S385. - PubMed
-
- Koopman C, Pelletier KR, Murray JF, et al. Stanford presenteeism scale: health status and employee productivity. J Occup Environ Med. 2002;44:14–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
