Microdialysis elevation of adenosine in the basal forebrain produces vigilance impairments in the rat psychomotor vigilance task
- PMID: 18853936
- PMCID: PMC2572744
Microdialysis elevation of adenosine in the basal forebrain produces vigilance impairments in the rat psychomotor vigilance task
Abstract
Study objective: The inhibitory neuromodulator adenosine has been proposed as a homeostatic sleep factor that acts potently in the basal forebrain (BF) to increase sleepiness. Here 300 microM of adenosine was dialyzed in the BF of rats, and the effect on vigilance was determined in the rat Psychomotor Vigilance Task (rPVT).
Design: Rats experienced all experimental conditions in a repeated-measures, cross-over design.
Patients or participants: Twelve young adult male Fischer-Norway rats.
Interventions: Sustained attention performance in the rPVT was evaluated following 2 hours of bilateral microdialysis perfusion of vehicle, adenosine (300 microM), or codialysis of 300 microM of adenosine with the A1 receptor antagonist 8-cyclopentyltheophylline.
Measurements and results: During rPVT performance, response latencies and performance lapses increased significantly after adenosine dialysis when compared with baseline (no dialysis) or vehicle dialysis sessions. The codialysis of 8-cyclopentyltheophylline with adenosine completely blocked the effects produced by adenosine alone, resulting in performance equivalent to that of the vehicle sessions.
Conclusions: Pharmacologic elevation of BF adenosine in rats produced vigilance impairments resembling the effect of sleep deprivation on vigilance performance in both man and rats. This effect of exogenous adenosine was completely blocked by codialysis with an adenosine A1 receptor antagonist. The results are consistent with the hypothesis that sleep loss induces elevations of BF adenosine that, acting via A1 receptors, lead to increased sleepiness and impaired vigilance.
Figures
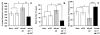
References
-
- McGaughy J, Sarter M. Sustained attention performance in rats with intracortical infusions of 192 IgG-saporin-induced cortical cholinergic deafferentation: effects of physostigmine and FG 7142. Behav Neurosci. 1998;112:1519–25. - PubMed
-
- Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol. 1999;9:178–83. - PubMed
-
- Detrain L, Rasmusson DD, Semba K. The role of basal forebrain neurons in tonic and phasic activation of the cerebral cortex. Prog Neurobiol. 1999;58:249–77. - PubMed
-
- Lin SC, Gervasoni D, Nicolelis MA. Fast modulation of prefrontal cortex activity by basal forebrain noncholinergic neuronal ensembles. J Neurophysiol. 2006;96:3209–19. - PubMed
-
- Manns ID, Alonso A, Jones BE. Rhythmically discharging basal forebrain units comprise cholinergic, GABAergic, and putative glutamatergic cells. J Neurophysiol. 2003;89:1057–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
