Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity
- PMID: 18854155
- PMCID: PMC2586330
- DOI: 10.1016/j.cell.2008.07.043
Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity
Abstract
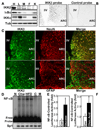
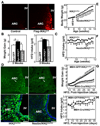
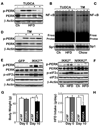
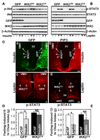
Overnutrition is associated with chronic inflammation in metabolic tissues. Whether metabolic inflammation compromises the neural regulatory systems and therefore promotes overnutrition-associated diseases remains unexplored. Here we show that a mediator of metabolic inflammation, IKKbeta/NF-kappaB, normally remains inactive although enriched in hypothalamic neurons. Overnutrition atypically activates hypothalamic IKKbeta/NF-kappaB at least in part through elevated endoplasmic reticulum stress in the hypothalamus. While forced activation of hypothalamic IKKbeta/NF-kappaB interrupts central insulin/leptin signaling and actions, site- or cell-specific suppression of IKKbeta either broadly across the brain or locally within the mediobasal hypothalamus, or specifically in hypothalamic AGRP neurons significantly protects against obesity and glucose intolerance. The molecular mechanisms involved include regulation by IKKbeta/NF-kappaB of SOCS3, a core inhibitor of insulin and leptin signaling. Our results show that the hypothalamic IKKbeta/NF-kappaB program is a general neural mechanism for energy imbalance underlying obesity and suggest that suppressing hypothalamic IKKbeta/NF-kappaB may represent a strategy to combat obesity and related diseases.
Figures


Comment in
-
Stressing the brain, fattening the body.Cell. 2008 Oct 3;135(1):20-2. doi: 10.1016/j.cell.2008.09.030. Cell. 2008. PMID: 18854151
References
-
- Arkan VC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. Nat. Med. 2005;11:191–198. - PubMed
-
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. - PubMed
-
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. - PubMed
-
- Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat. Med. 2006;12:917–924. - PubMed
-
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

