Behavioral recovery from acute hypoxia is reliant on leptin
- PMID: 18854211
- PMCID: PMC2652853
- DOI: 10.1016/j.bbi.2008.09.011
Behavioral recovery from acute hypoxia is reliant on leptin
Abstract
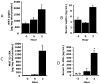
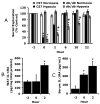
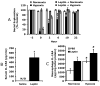
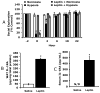
Individuals affected by hypoxia experience a variety of immune-associated sickness symptoms including malaise, fatigue, lethargy and loss of interest in the physical and social environment. Recently, we demonstrated that the interleukin (IL)-1beta arm of the neuroimmune system was critical to the sickness symptoms caused by hypoxia, and that IL-1 receptor antagonist (IL-1RA), IL-1beta's endogenous inhibitor, was critical to promoting sickness recovery. Here, we report that leptin is key to recovery from hypoxia because it dramatically augmented IL-1RA production in mice. We found that hypoxia increased leptin in white adipose tissue (WAT) which in turn, caused a marked rise in serum IL-1RA. Interestingly, in-vitro, leptin was a more potent inducer of IL-RA, in macrophages, than hypoxia. In leptin receptor defective (db/db) and leptin deficient (ob/ob) mice, sickness recovery from hypoxia was delayed 3-fold. Importantly, in ob/ob mice, leptin administration completely reversed this delayed recovery and induced a marked increase in serum IL-1RA. Finally, leptin administration to normal mice reduced hypoxia recovery time by 1/3 and dramatically increased WAT and serum IL-1RA. Leptin did not alter recovery from hypoxia in IL-1RA knock out mice. These results show that by enhancing IL-1RA production leptin promoted sickness recovery from hypoxia.
Figures



Comment in
-
Ob or not ob? Is leptin involved in hypoxia-induced social withdrawal?Brain Behav Immun. 2009 Feb;23(2):167-8. doi: 10.1016/j.bbi.2008.11.001. Epub 2008 Nov 11. Brain Behav Immun. 2009. PMID: 19041392 No abstract available.
References
-
- Ambrosini G, Nath AK, Sierra-Honigmann MR, Flores-Riveros J. Transcriptional activation of the human leptin gene in response to hypoxia. Involvement of hypoxia-inducible factor 1. J Biol Chem. 2002;277:34601–34609. - PubMed
-
- Basnyat B, Murdoch DR. High-altitude illness. Lancet. 2003;361:1967–1974. - PubMed
-
- Basu A, Lazovic J, Krady JK, Mauger DT, Rothstein RP, Smith MB, Levison SW. Interleukin-1 and the interleukin-1 type 1 receptor are essential for the progressive neurodegeneration that ensues subsequent to a mild hypoxic/ischemic injury. J Cereb Blood Flow Metab. 2005;25:17–29. - PubMed
-
- Braunwald E. Hypoxia and Cyanosis. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JK, Loscalzo JL, editors. Harrison’s Principles of Internal Medicine. McGraw Hill Medical, United States of American; 2008. pp. 230–231.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

