DYRK3 dual-specificity kinase attenuates erythropoiesis during anemia
- PMID: 18854306
- PMCID: PMC2606001
- DOI: 10.1074/jbc.M807844200
DYRK3 dual-specificity kinase attenuates erythropoiesis during anemia
Abstract
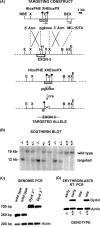
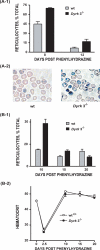
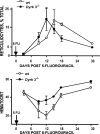
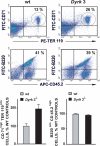
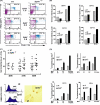
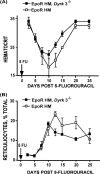
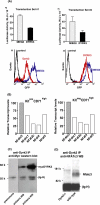
During anemia erythropoiesis is bolstered by several factors including KIT ligand, oncostatin-M, glucocorticoids, and erythropoietin. Less is understood concerning factors that limit this process. Experiments performed using dual-specificity tyrosine-regulated kinase-3 (DYRK3) knock-out and transgenic mice reveal that erythropoiesis is attenuated selectively during anemia. DYRK3 is restricted to erythroid progenitor cells and testes. DYRK3-/- mice exhibited essentially normal hematological profiles at steady state and reproduced normally. In response to hemolytic anemia, however, reticulocyte production increased severalfold due to DYRK3 deficiency. During 5-fluorouracil-induced anemia, both reticulocyte and red cell formation in DYRK3-/- mice were elevated. In short term transplant experiments, DYRK3-/- progenitors also supported enhanced erythroblast formation, and erythropoietic advantages due to DYRK3-deficiency also were observed in 5-fluorouracil-treated mice expressing a compromised erythropoietin receptor EPOR-HM allele. As analyzed ex vivo, DYRK3-/- erythroblasts exhibited enhanced CD71posTer119pos cell formation and 3HdT incorporation. Transgenic pA2gata1-DYRK3 mice, in contrast, produced fewer reticulocytes during hemolytic anemia, and pA2gata1-DYRK3 progenitors were compromised in late pro-erythroblast formation ex vivo. Finally, as studied in erythroid K562 cells, DYRK3 proved to effectively inhibit NFAT (nuclear factor of activated T cells) transcriptional response pathways and to co-immunoprecipitate with NFATc3. Findings indicate that DYRK3 attenuates (and possibly apportions) red cell production selectively during anemia.
Figures

Similar articles
-
Signals for stress erythropoiesis are integrated via an erythropoietin receptor-phosphotyrosine-343-Stat5 axis.J Clin Invest. 2006 Mar;116(3):683-94. doi: 10.1172/JCI25227. J Clin Invest. 2006. PMID: 16511603 Free PMC article.
-
A KIT juxtamembrane PY567 -directed pathway provides nonredundant signals for erythroid progenitor cell development and stress erythropoiesis.Exp Hematol. 2009 Feb;37(2):159-71. doi: 10.1016/j.exphem.2008.10.009. Epub 2008 Dec 18. Exp Hematol. 2009. PMID: 19100679 Free PMC article.
-
Erythropoietin-dependent erythropoiesis: New insights and questions.Blood Cells Mol Dis. 2006 Mar-Apr;36(2):232-8. doi: 10.1016/j.bcmd.2006.01.007. Epub 2006 Mar 9. Blood Cells Mol Dis. 2006. PMID: 16524748 Review.
-
Spry1 as a novel regulator of erythropoiesis, EPO/EPOR target, and suppressor of JAK2.Blood. 2012 Jun 7;119(23):5522-31. doi: 10.1182/blood-2011-11-392571. Epub 2012 Apr 16. Blood. 2012. PMID: 22508938 Free PMC article.
-
[Expression and extracellular release of transferrin receptors on erythropoiesis].Rinsho Ketsueki. 1991 Jun;32(6):580-6. Rinsho Ketsueki. 1991. PMID: 1890732 Review. Japanese.
Cited by
-
New insights into the roles for DYRK family in mammalian development and congenital diseases.Genes Dis. 2022 Jan 6;10(3):758-770. doi: 10.1016/j.gendis.2021.12.004. eCollection 2023 May. Genes Dis. 2022. PMID: 37396550 Free PMC article. Review.
-
Functions of SRPK, CLK and DYRK kinases in stem cells, development, and human developmental disorders.FEBS Lett. 2023 Oct;597(19):2375-2415. doi: 10.1002/1873-3468.14723. Epub 2023 Sep 4. FEBS Lett. 2023. PMID: 37607329 Free PMC article. Review.
-
Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells is mediated through the lipid rafts-endocytic pathway via the dual specificity tyrosine phosphorylation-regulated kinase 3 (DYRK3).J Biol Chem. 2011 Oct 7;286(40):34761-9. doi: 10.1074/jbc.M111.219378. Epub 2011 Jun 21. J Biol Chem. 2011. PMID: 21693704 Free PMC article.
-
Active site coupling in PDE:PKA complexes promotes resetting of mammalian cAMP signaling.Biophys J. 2014 Sep 16;107(6):1426-40. doi: 10.1016/j.bpj.2014.07.050. Biophys J. 2014. PMID: 25229150 Free PMC article.
-
Death associated protein kinase 2 is expressed in cortical interstitial cells of the mouse kidney.BMC Res Notes. 2014 Jun 7;7:345. doi: 10.1186/1756-0500-7-345. BMC Res Notes. 2014. PMID: 24906443 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

