Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma
- PMID: 18854392
- PMCID: PMC2630877
- DOI: 10.1210/jc.2008-1456
Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma
Abstract
Context: Drug therapy for adrenocortical carcinoma (ACC), a rare and lethal malignancy, is largely empirical and ineffective. New treatments directed at molecular targets critical to the pathophysiology of ACC may prove more efficacious.
Objective: The objective of the study was to profile human adrenal tumors and ACC cell lines to assess activated IGF signaling and determine the efficacy of two IGF receptor (IGF-1R) antagonists alone and in combination with mitotane.
Experimental design: ACC cell lines that display or lack activated IGF signaling are used to assess the effects of two IGF-1R antagonists in cultured cells and ACC xenograft tumors.
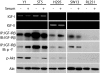
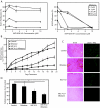
Results: Transcriptional profiling data derived from DNA microarray analysis of human adrenal tumors implicate IGF2 as the single highest up-regulated transcript in the vast majority of carcinomas. We show that the majority of ACC cell lines tested display constitutive IGF ligand production and activation of downstream effector pathways. Both IGF-1R antagonists cause significant dose-dependent growth inhibition in ACC cell lines. Furthermore, we observe that mitotane, the first-line adrenolytic drug used in patients with ACC, results in enhanced growth inhibition when used in combination with the IGF-1R antagonists. We next examined the activity of IGF-1R antagonists against ACC xenografts in athymic nude mice. IGF inhibition markedly reduced tumor growth greater than that observed with mitotane treatment, and combination therapy with mitotane significantly enhanced tumor growth suppression.
Conclusion: These findings establish a critical role of IGF signaling in ACC pathophysiology and provide rationale for use of targeted IGF-1R antagonists to treat adrenocortical carcinoma in future clinical trials.
Figures


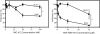

References
-
- Wajchenberg BL, Albergaria Pereira MA, Medonca BB, Latronico AC, Campos Carneiro P, Alves VA, Zerbini MC, Liberman B, Carlos Gomes G, Kirschner MA 2000 Adrenocortical carcinoma: clinical and laboratory observations. Cancer 88:711–736 - PubMed
-
- Allolio B, Fassnacht M 2006 Clinical review: adrenocortical carcinoma: clinical update. J Clin Endocrinol Metab 91:2027–2037 - PubMed
-
- Icard P, Goudet P, Charpenay C, Andreassian B, Carnaille B, Chapuis Y, Cougard P, Henry JF, Proye C 2001 Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons Study Group. World J Surg 25:891–897 - PubMed
-
- Velazquez-Fernandez D, Laurell C, Geli J, Hoog A, Odeberg J, Kjellman M, Lundeberg J, Hamberger B, Nilsson P, Backdahl M 2005 Expression profiling of adrenocortical neoplasms suggests a molecular signature of malignancy. Surgery 138:1087–1094 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

