GPIHBP1, a GPI-anchored protein required for the lipolytic processing of triglyceride-rich lipoproteins
- PMID: 18854402
- PMCID: PMC2674691
- DOI: 10.1194/jlr.R800030-JLR200
GPIHBP1, a GPI-anchored protein required for the lipolytic processing of triglyceride-rich lipoproteins
Abstract
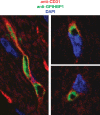
GPIHBP1, a small glycosylphosphatidylinositol-anchored glycoprotein, is required for the lipolytic processing of triglyceride-rich lipoproteins. GPIHBP1 knockout mice exhibit chylomicronemia, even on a low-fat diet, with plasma triglyceride levels of 3,500-5,000 mg/dl. GPIHBP1 is expressed highly in heart, adipose tissue, and skeletal muscle, the same tissues that express high levels of lipoprotein lipase (LPL). In each of these tissues, GPIHBP1 is located in capillary endothelial cells. Chinese hamster ovary (CHO) cells transfected with a GPIHBP1 expression vector bind LPL and chylomicrons avidly. The expression of GPIHBP1 in mice is modulated by fasting and refeeding and is also regulated by peroxisome proliferator-activated receptor (PPAR)gamma agonists. Here, we review recent progress in understanding GPIHBP1 and discuss its role in lipolysis.
Figures
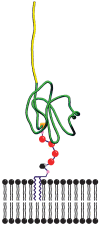

Similar articles
-
GPIHBP1, an endothelial cell transporter for lipoprotein lipase.J Lipid Res. 2011 Nov;52(11):1869-84. doi: 10.1194/jlr.R018689. Epub 2011 Aug 15. J Lipid Res. 2011. PMID: 21844202 Free PMC article. Review.
-
GPIHBP1 and lipolysis: an update.Curr Opin Lipidol. 2009 Jun;20(3):211-6. doi: 10.1097/MOL.0b013e32832ac026. Curr Opin Lipidol. 2009. PMID: 19369870 Free PMC article. Review.
-
GPIHBP1: an endothelial cell molecule important for the lipolytic processing of chylomicrons.Curr Opin Lipidol. 2007 Aug;18(4):389-96. doi: 10.1097/MOL.0b013e3281527914. Curr Opin Lipidol. 2007. PMID: 17620854 Free PMC article. Review.
-
The expression of GPIHBP1, an endothelial cell binding site for lipoprotein lipase and chylomicrons, is induced by peroxisome proliferator-activated receptor-gamma.Mol Endocrinol. 2008 Nov;22(11):2496-504. doi: 10.1210/me.2008-0146. Epub 2008 Sep 11. Mol Endocrinol. 2008. PMID: 18787041 Free PMC article.
-
Chylomicronemia with low postheparin lipoprotein lipase levels in the setting of GPIHBP1 defects.Circ Cardiovasc Genet. 2010 Apr;3(2):169-78. doi: 10.1161/CIRCGENETICS.109.908905. Epub 2010 Feb 2. Circ Cardiovasc Genet. 2010. PMID: 20124439 Free PMC article.
Cited by
-
Identification and quantitative mRNA analysis of a novel splice variant of GPIHBP1 in dairy cattle.J Anim Sci Biotechnol. 2014 Nov 5;5(1):50. doi: 10.1186/2049-1891-5-50. eCollection 2014. J Anim Sci Biotechnol. 2014. PMID: 25810903 Free PMC article.
-
Influence of apolipoprotein A-V on the metabolic fate of triacylglycerol.Curr Opin Lipidol. 2013 Apr;24(2):153-9. doi: 10.1097/MOL.0b013e32835c8c1a. Curr Opin Lipidol. 2013. PMID: 23241513 Free PMC article. Review.
-
Effects of the prosegment and pH on the activity of PCSK9: evidence for additional processing events.J Biol Chem. 2010 Dec 24;285(52):40965-78. doi: 10.1074/jbc.M110.154815. Epub 2010 Oct 11. J Biol Chem. 2010. PMID: 20937814 Free PMC article.
-
An anti-ANGPTL3/8 antibody decreases circulating triglycerides by binding to a LPL-inhibitory leucine zipper-like motif.J Lipid Res. 2022 May;63(5):100198. doi: 10.1016/j.jlr.2022.100198. Epub 2022 Mar 17. J Lipid Res. 2022. PMID: 35307397 Free PMC article.
-
GPIHBP1, an endothelial cell transporter for lipoprotein lipase.J Lipid Res. 2011 Nov;52(11):1869-84. doi: 10.1194/jlr.R018689. Epub 2011 Aug 15. J Lipid Res. 2011. PMID: 21844202 Free PMC article. Review.
References
-
- Korn E. D. 1955. Clearing factor, a heparin-activated lipoprotein lipase. II. Substrate specificity and activation of coconut oil. J. Biol. Chem. 215 15–26. - PubMed
-
- Brunzell, J. D., and S. S. Deeb. 2001. Familial lipoprotein lipase deficiency, apo C–II deficiency, and hepatic lipase deficiency. In The Metabolic and Molecular Bases of Inherited Disease. C. R. Scriver, A. L. Beaudet, W. S. Sly, D. Valle, B. Childs, K. W. Kinzler, and B. Vogelstein, editors. McGraw-Hill, New York. 2789–2816.
-
- Havel, R. J., and J. P. Kane. 2001. Introduction: Structure and metabolism of plasma lipoproteins. In The Metabolic and Molecular Bases of Inherited Disease. C. R. Scriver, A. L. Beaudet, W. S. Sly, D. Valle, B. Childs, K. W. Kinzler, and B. Vogelstein, editors. McGraw-Hill, New York. 2705–2716.
-
- Tan M. H., T. Sata, and R. J. Havel. 1977. The significance of lipoprotein lipase in rat skeletal muscles. J. Lipid Res. 18 363–370. - PubMed
-
- Inaba T., M. Matsuda, M. Shimamura, N. Takei, N. Terasaka, Y. Ando, H. Yasumo, R. Koishi, M. Makishima, and I. Shimomura. 2003. Angiopoietin-like protein 3 mediates hypertriglyceridemia induced by the liver X receptor. J. Biol. Chem. 278 21344–21351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

