Azatryptophans endow proteins with intrinsic blue fluorescence
- PMID: 18854410
- PMCID: PMC2571030
- DOI: 10.1073/pnas.0802804105
Azatryptophans endow proteins with intrinsic blue fluorescence
Abstract
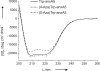
Our long-term goal is the in vivo expression of intrinsically colored proteins without the need for further posttranslational modification or chemical functionalization by externally added reagents. Biocompatible (Aza)Indoles (Inds)/(Aza)Tryptophans (Trp) as optical probes represent almost ideal isosteric substitutes for natural Trp in cellular proteins. To overcome the limits of the traditionally used (7-Aza)Ind/(7-Aza)Trp, we substituted the single Trp residue in human annexin A5 (anxA5) by (4-Aza)Trp and (5-Aza)Trp in Trp-auxotrophic Escherichia coli cells. Both cells and proteins with these fluorophores possess intrinsic blue fluorescence detectable on routine UV irradiations. We identified (4-Aza)Ind as a superior optical probe due to its pronounced Stokes shift of approximately 130 nm, its significantly higher quantum yield (QY) in aqueous buffers and its enhanced quenching resistance. Intracellular metabolic transformation of (4-Aza)Ind into (4-Aza)Trp coupled with high yield incorporation into proteins is the most straightforward method for the conversion of naturally colorless proteins and cells into their blue counterparts from amino acid precursors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Blue fluorescent amino acids as in vivo building blocks for proteins.Chembiochem. 2010 Feb 15;11(3):305-14. doi: 10.1002/cbic.200900651. Chembiochem. 2010. PMID: 20058252
-
Incorporation of beta-selenolo[3,2-b]pyrrolyl-alanine into proteins for phase determination in protein X-ray crystallography.J Mol Biol. 2001 Jun 15;309(4):925-36. doi: 10.1006/jmbi.2001.4699. J Mol Biol. 2001. PMID: 11399069
-
Role of Trp-187 in the annexin V-membrane interaction: a molecular mechanics analysis.Biochem Biophys Res Commun. 1999 Jan 19;254(2):484-9. doi: 10.1006/bbrc.1998.9965. Biochem Biophys Res Commun. 1999. PMID: 9918865
-
Tryptophan fluorescence quenching by methionine and selenomethionine residues of calmodulin: orientation of peptide and protein binding.Biochemistry. 1998 Mar 3;37(9):3187-95. doi: 10.1021/bi9716579. Biochemistry. 1998. PMID: 9485473
-
Atomic mutations at the single tryptophan residue of human recombinant annexin V: effects on structure, stability, and activity.Biochemistry. 1999 Aug 17;38(33):10649-59. doi: 10.1021/bi990580g. Biochemistry. 1999. PMID: 10451359
Cited by
-
Importance of single molecular determinants in the fidelity of expanded genetic codes.Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1320-5. doi: 10.1073/pnas.1012276108. Epub 2011 Jan 11. Proc Natl Acad Sci U S A. 2011. PMID: 21224416 Free PMC article.
-
N-Terminally extended analogues of the K⁺ channel toxin from Stichodactyla helianthus as potent and selective blockers of the voltage-gated potassium channel Kv1.3.FEBS J. 2015 Jun;282(12):2247-59. doi: 10.1111/febs.13294. Epub 2015 Apr 23. FEBS J. 2015. PMID: 25864722 Free PMC article.
-
Efficient asymmetric synthesis of tryptophan analogues having useful photophysical properties.Org Lett. 2014 Jan 17;16(2):556-9. doi: 10.1021/ol403429e. Epub 2014 Jan 6. Org Lett. 2014. PMID: 24392870 Free PMC article.
-
Synthesis of 2-substituted tryptophans via a C3- to C2-alkyl migration.Beilstein J Org Chem. 2014 Aug 26;10:1991-8. doi: 10.3762/bjoc.10.207. eCollection 2014. Beilstein J Org Chem. 2014. PMID: 25246958 Free PMC article.
-
Synthesis and application of the blue fluorescent amino acid l-4-cyanotryptophan to assess peptide-membrane interactions.Chem Commun (Camb). 2019 Apr 25;55(35):5095-5098. doi: 10.1039/c9cc01152h. Chem Commun (Camb). 2019. PMID: 30957824 Free PMC article.
References
-
- Lakowitz JR. Protein Fluorescence. New York: Kluwer Academic/Plenum; 1999.
-
- Budisa N, et al. Probing the role of tryptophans in Aequorea victoria green fluorescent proteins with an expanded genetic code. Biol Chem. 2004;385:191–202. - PubMed
-
- Ross JBA, Szabo AG, Hogue CWV. Fluorescence Spectroscopy. 1997;Vol 278:151–190. - PubMed
-
- Minks C, Huber R, Moroder L, Budisa N. Atomic mutations at the single tryptophan residue of human recombinant annexin V: Effects on structure, stability, and activity. Biochemistry. 1999;38:10649–10659. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

