Gene therapy using adeno-associated virus vectors
- PMID: 18854481
- PMCID: PMC2570152
- DOI: 10.1128/CMR.00008-08
Gene therapy using adeno-associated virus vectors
Abstract
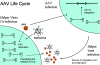
The unique life cycle of adeno-associated virus (AAV) and its ability to infect both nondividing and dividing cells with persistent expression have made it an attractive vector. An additional attractive feature of the wild-type virus is the lack of apparent pathogenicity. Gene transfer studies using AAV have shown significant progress at the level of animal models; clinical trials have been noteworthy with respect to the safety of AAV vectors. No proven efficacy has been observed, although in some instances, there have been promising observations. In this review, topics in AAV biology are supplemented with a section on AAV clinical trials with emphasis on the need for a deeper understanding of AAV biology and the development of efficient AAV vectors. In addition, several novel approaches and recent findings that promise to expand AAV's utility are discussed, especially in the context of combining gene therapy ex vivo with new advances in stem or progenitor cell biology.
Figures


References
-
- Aitken, M. L., R. B. Moss, D. A. Waltz, M. E. Dovey, M. R. Tonelli, S. C. McNamara, R. L. Gibson, B. W. Ramsey, B. J. Carter, and T. C. Reynolds. 2001. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum. Gene Ther. 12:1907-1916. - PubMed
-
- Anand, V., B. Duffy, Z. Yang, N. S. Dejneka, A. M. Maguire, and J. Bennett. 2002. A deviant immune response to viral proteins and transgene product is generated on subretinal administration of adenovirus and adeno-associated virus. Mol. Ther. 5:125-132. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

