High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial
- PMID: 18854539
- PMCID: PMC2684821
- DOI: 10.1001/jama.300.15.1774
High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial
Abstract
Context: Blood levels of homocysteine may be increased in Alzheimer disease (AD) and hyperhomocysteinemia may contribute to disease pathophysiology by vascular and direct neurotoxic mechanisms. Even in the absence of vitamin deficiency, homocysteine levels can be reduced by administration of high-dose supplements of folic acid and vitamins B(6) and B(12). Prior studies of B vitamins to reduce homocysteine in AD have not had sufficient size or duration to assess their effect on cognitive decline.
Objective: To determine the efficacy and safety of B vitamin supplementation in the treatment of AD.
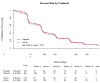
Design, setting, and patients: A multicenter, randomized, double-blind controlled clinical trial of high-dose folate, vitamin B(6), and vitamin B(12) supplementation in 409 (of 601 screened) individuals with mild to moderate AD (Mini-Mental State Examination scores between 14 and 26, inclusive) and normal folic acid, vitamin B(12), and homocysteine levels. The study was conducted between February 20, 2003, and December 15, 2006, at clinical research sites of the Alzheimer Disease Cooperative Study located throughout the United States.
Intervention: Participants were randomly assigned to 2 groups of unequal size to increase enrollment (60% treated with high-dose supplements [5 mg/d of folate, 25 mg/d of vitamin B(6), 1 mg/d of vitamin B(12)] and 40% treated with identical placebo); duration of treatment was 18 months.
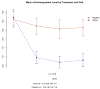
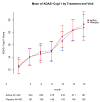
Main outcome measure: Change in the cognitive subscale of the Alzheimer Disease Assessment Scale (ADAS-cog).
Results: A total of 340 participants (202 in active treatment group and 138 in placebo group) completed the trial while taking study medication. Although the vitamin supplement regimen was effective in reducing homocysteine levels (mean [SD], -2.42 [3.35] in active treatment group vs -0.86 [2.59] in placebo group; P < .001), it had no beneficial effect on the primary cognitive measure, rate of change in ADAS-cog score during 18 months (0.372 points per month for placebo group vs 0.401 points per month for active treatment group, P = .52; 95% confidence interval of rate difference, -0.06 to 0.12; based on the intention-to-treat generalized estimating equations model), or on any secondary measures. A higher quantity of adverse events involving depression was observed in the group treated with vitamin supplements.
Conclusion: This regimen of high-dose B vitamin supplements does not slow cognitive decline in individuals with mild to moderate AD.
Trial registration: clinicaltrials.gov Identifier: NCT00056225.
Figures


Comment in
-
B vitamins for prevention of cognitive decline: insufficient evidence to justify treatment.JAMA. 2008 Oct 15;300(15):1819-21. doi: 10.1001/jama.300.15.1819. JAMA. 2008. PMID: 18854547 No abstract available.
-
ACP Journal Club. High-dose vitamin B supplements did not slow cognitive decline in mild-to-moderate Alzheimer disease.Ann Intern Med. 2009 Feb 17;150(4):JC2-7. doi: 10.7326/0003-4819-150-4-200902170-02007. Ann Intern Med. 2009. PMID: 19238605 No abstract available.
-
High-dose B vitamin supplements and Alzheimer disease.JAMA. 2009 Mar 11;301(10):1020-1; author reply 1021-2. doi: 10.1001/jama.2009.211. JAMA. 2009. PMID: 19278942 No abstract available.
-
High-dose B vitamin supplements and Alzheimer disease.JAMA. 2009 Mar 11;301(10):1021; author reply 1021-2. doi: 10.1001/jama.2009.212. JAMA. 2009. PMID: 19278943 No abstract available.
-
High-dose vitamin B supplements did not slow cognitive decline in patients with mild-to-moderate Alzheimer disease.Evid Based Nurs. 2009 Apr;12(2):57. doi: 10.1136/ebn.12.2.57. Evid Based Nurs. 2009. PMID: 19321834 No abstract available.
-
High-dose B vitamin supplementation as a disease-modifying therapy in Alzheimer disease.Arch Neurol. 2009 Apr;66(4):520-2. doi: 10.1001/archneurol.2009.12. Arch Neurol. 2009. PMID: 19364938 No abstract available.
-
High dose vitamin B supplementation does not slow cognitive decline in mild to moderate Alzheimer's disease.Evid Based Ment Health. 2009 Aug;12(3):86. doi: 10.1136/ebmh.12.3.86. Evid Based Ment Health. 2009. PMID: 19633254 No abstract available.
References
-
- Aisen PS. The development of anti-amyloid therapy for Alzheimer’s disease : from secretase modulators to polymerisation inhibitors. CNS Drugs. 2005;19(12):989–996. - PubMed
-
- Fillit HM, Refolo LM. Tau and Alzheimer’s disease: the long road to anti-tangle therapeutics. J Mol Neurosci. 2002 Dec;19(3):249–250. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

