Futile Na+ cycling at the root plasma membrane in rice (Oryza sativa L.): kinetics, energetics, and relationship to salinity tolerance
- PMID: 18854575
- PMCID: PMC2639017
- DOI: 10.1093/jxb/ern249
Futile Na+ cycling at the root plasma membrane in rice (Oryza sativa L.): kinetics, energetics, and relationship to salinity tolerance
Abstract
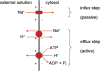
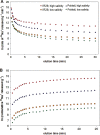
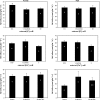
Globally, over one-third of irrigated land is affected by salinity, including much of the land under lowland rice cultivation in the tropics, seriously compromising yields of this most important of crop species. However, there remains an insufficient understanding of the cellular basis of salt tolerance in rice. Here, three methods of 24Na+ tracer analysis were used to investigate primary Na+ transport at the root plasma membrane in a salt-tolerant rice cultivar (Pokkali) and a salt-sensitive cultivar (IR29). Futile cycling of Na+ at the plasma membrane of intact roots occurred at both low and elevated levels of steady-state Na+ supply ([Na+]ext=1 mM and 25 mM) in both cultivars. At 25 mM [Na+]ext, a toxic condition for IR29, unidirectional influx and efflux of Na+ in this cultivar, but not in Pokkali, became very high [>100 micromol g (root FW)(-1) h(-1)], demonstrating an inability to restrict sodium fluxes. Current models of sodium transport energetics across the plasma membrane in root cells predict that, if the sodium efflux were mediated by Na+/H+ antiport, this toxic scenario would impose a substantial respiratory cost in IR29. This cost is calculated here, and compared with root respiration, which, however, comprised only approximately 50% of what would be required to sustain efflux by the antiporter. This suggests that either the conventional 'leak-pump' model of Na+ transport or the energetic model of proton-linked Na+ transport may require some revision. In addition, the lack of suppression of Na+ influx by both K+ and Ca2+, and by the application of the channel inhibitors Cs+, TEA+, and Ba2+, questions the participation of potassium channels and non-selective cation channels in the observed Na+ fluxes.
Figures



References
-
- Amtmann A, Gradmann D. Na+ transport in Acetabularia bypasses conductance of plasmalemma. Journal of Membrane Biology. 1994;139:117–125. - PubMed
-
- Anil VS, Krishnamurthy P, Kuruvilla S, Sucharitha K, Thomas G, Mathew MK. Regulation of the uptake and distribution of Na+ in shoots of rice (Oryza sativa) variety Pokkali: role of Ca2+ in salt tolerance response. Physiologia Plantarum. 2005;124:451–464.
-
- Apse MP, Aharon GS, Snedden WA, Blumwald E. Overexpression of a vacuolar Na+/H+ antiport confers salt tolerance in Arabidopsis. Science. 1999;285:1256–1258. - PubMed
-
- Apse MP, Blumwald E. Na+ transport in plants. FEBS Letters. 2007;581:2247–2254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

