Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease
- PMID: 18854702
- PMCID: PMC3731075
- DOI: 10.1097/EDE.0b013e3181875e61
Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease
Abstract
Background: The Women's Health Initiative randomized trial found greater coronary heart disease (CHD) risk in women assigned to estrogen/progestin therapy than in those assigned to placebo. Observational studies had previously suggested reduced CHD risk in hormone users.
Methods: Using data from the observational Nurses' Health Study, we emulated the design and intention-to-treat (ITT) analysis of the randomized trial. The observational study was conceptualized as a sequence of "trials," in which eligible women were classified as initiators or noninitiators of estrogen/progestin therapy.
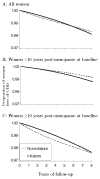
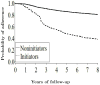
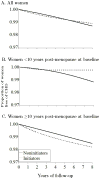
Results: The ITT hazard ratios (HRs) (95% confidence intervals) of CHD for initiators versus noninitiators were 1.42 (0.92-2.20) for the first 2 years, and 0.96 (0.78-1.18) for the entire follow-up. The ITT HRs were 0.84 (0.61-1.14) in women within 10 years of menopause, and 1.12 (0.84-1.48) in the others (P value for interaction = 0.08). These ITT estimates are similar to those from the Women's Health Initiative. Because the ITT approach causes severe treatment misclassification, we also estimated adherence-adjusted effects by inverse probability weighting. The HRs were 1.61 (0.97-2.66) for the first 2 years, and 0.98 (0.66-1.49) for the entire follow-up. The HRs were 0.54 (0.19-1.51) in women within 10 years after menopause, and 1.20 (0.78-1.84) in others (P value for interaction = 0.01). We also present comparisons between these estimates and previously reported Nurses' Health Study estimates.
Conclusions: Our findings suggest that the discrepancies between the Women's Health Initiative and Nurses' Health Study ITT estimates could be largely explained by differences in the distribution of time since menopause and length of follow-up.
Figures
Comment in
-
Data analysis methods and the reliability of analytic epidemiologic research.Epidemiology. 2008 Nov;19(6):785-8; discussion 789-93. doi: 10.1097/EDE.0b013e318188e83b. Epidemiology. 2008. PMID: 18813015 Free PMC article.
-
The sound and the fury: was it all worth it?Epidemiology. 2008 Nov;19(6):780-2; discussion 789-93. doi: 10.1097/EDE.0b013e318188e21d. Epidemiology. 2008. PMID: 18813016
-
ITT for observational data: worst of both worlds?Epidemiology. 2008 Nov;19(6):783-4; discussion 789-93. doi: 10.1097/EDE.0b013e318188442e. Epidemiology. 2008. PMID: 18813017
References
-
- Ioannidis JP, Haidich AB, Pappa M, et al. Comparison of evidence of treatment effects in randomized and nonrandomized studies. Jama. 2001;286(7):821–30. - PubMed
-
- Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342(25):1878–86. - PubMed
-
- Grodstein F, Stampfer M, Manson J, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease (Erratum in: N Engl J Med 1996;335:1406) New England Journal of Medicine. 1996;335(7):453–61. - PubMed
-
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Annals of Internal Medicine. 2000;133(12):933–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

