Late sodium current is a new therapeutic target to improve contractility and rhythm in failing heart
- PMID: 18855648
- PMCID: PMC2575131
- DOI: 10.2174/187152508785909447
Late sodium current is a new therapeutic target to improve contractility and rhythm in failing heart
Abstract
Most cardiac Na+ channels open transiently within milliseconds upon membrane depolarization and are responsible for the excitation propagation. However, some channels remain active during hundreds of milliseconds, carrying the so-called persistent or late Na+ current (I(NaL)) throughout the action potential plateau. I(NaL) is produced by special gating modes of the cardiac-specific Na+ channel isoform. Experimental data accumulated over the past decade show the emerging importance of this late current component for the function of both normal and especially failing myocardium, where I(NaL) is reportedly increased. Na+ channels represent a multi-protein complex and its activity is determined not only by the pore-forming alpha subunit but also by its auxiliary beta subunits, cytoskeleton, and by Ca2+ signaling and trafficking proteins. Remodeling of this protein complex and intracellular signaling pathways may lead to alterations of I(NaL) in pathological conditions. Increased I(NaL) and the corresponding Na+ influx in failing myocardium contribute to abnormal repolarization and an increased cell Ca2+ load. Interventions designed to correct I(NaL) rescue normal repolarization and improve Ca2+ handling and contractility of the failing cardiomyocytes. New therapeutic strategies to target both arrhythmias and deficient contractility in HF may not be limited to the selective inhibition of I(NaL) but also include multiple indirect, modulatory (e.g. Ca(2+)- or cytoskeleton- dependent) mechanisms of I(NaL) function.
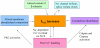
Figures



Similar articles
-
Late sodium current in failing heart: friend or foe?Prog Biophys Mol Biol. 2008 Jan-Apr;96(1-3):421-51. doi: 10.1016/j.pbiomolbio.2007.07.010. Epub 2007 Aug 10. Prog Biophys Mol Biol. 2008. PMID: 17854868 Free PMC article. Review.
-
Modulation of late sodium current by Ca2+, calmodulin, and CaMKII in normal and failing dog cardiomyocytes: similarities and differences.Am J Physiol Heart Circ Physiol. 2008 Apr;294(4):H1597-608. doi: 10.1152/ajpheart.00484.2007. Epub 2008 Jan 18. Am J Physiol Heart Circ Physiol. 2008. PMID: 18203851 Free PMC article.
-
Late Na+ current produced by human cardiac Na+ channel isoform Nav1.5 is modulated by its beta1 subunit.J Physiol Sci. 2009 May;59(3):217-25. doi: 10.1007/s12576-009-0029-7. Epub 2009 Mar 3. J Physiol Sci. 2009. PMID: 19340536 Free PMC article.
-
Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability.Eur J Heart Fail. 2007 Mar;9(3):219-27. doi: 10.1016/j.ejheart.2006.08.007. Epub 2006 Oct 24. Eur J Heart Fail. 2007. PMID: 17067855 Free PMC article.
-
Pathophysiology of the cardiac late Na current and its potential as a drug target.J Mol Cell Cardiol. 2012 Mar;52(3):608-19. doi: 10.1016/j.yjmcc.2011.12.003. Epub 2011 Dec 16. J Mol Cell Cardiol. 2012. PMID: 22198344 Free PMC article. Review.
Cited by
-
Neuronal sodium channels: emerging components of the nano-machinery of cardiac calcium cycling.J Physiol. 2017 Jun 15;595(12):3823-3834. doi: 10.1113/JP273058. Epub 2017 Mar 26. J Physiol. 2017. PMID: 28195313 Free PMC article. Review.
-
Ranolazine: An Old Drug with Emerging Potential; Lessons from Pre-Clinical and Clinical Investigations for Possible Repositioning.Pharmaceuticals (Basel). 2021 Dec 25;15(1):31. doi: 10.3390/ph15010031. Pharmaceuticals (Basel). 2021. PMID: 35056088 Free PMC article. Review.
-
Late sodium current (INaL) in pancreatic β-cells.Pflugers Arch. 2015 Aug;467(8):1757-68. doi: 10.1007/s00424-014-1613-0. Epub 2014 Sep 20. Pflugers Arch. 2015. PMID: 25236919
-
Contribution of sodium channel neuronal isoform Nav1.1 to late sodium current in ventricular myocytes from failing hearts.J Physiol. 2015 Mar 15;593(6):1409-27. doi: 10.1113/jphysiol.2014.278259. Epub 2014 Nov 17. J Physiol. 2015. PMID: 25772296 Free PMC article.
-
Cardiac Arrhythmias as Manifestations of Nanopathies: An Emerging View.Front Physiol. 2018 Sep 4;9:1228. doi: 10.3389/fphys.2018.01228. eCollection 2018. Front Physiol. 2018. PMID: 30233404 Free PMC article. Review.
References
-
- Bayes de Luna A, Coumel P, Leclercq JF. Am Heart J. 1989;117:151. - PubMed
-
- Nikolic G, Bishop RL, Singh JB. Circulation. 1982;66:218. - PubMed
-
- Tomaselli GF, Zipes DP. Circ Res. 2004;95:754. - PubMed
-
- Bers DM, Despa S, Bossuyt J. Ann N Y Acad Sci. 2006;1080:165. - PubMed
-
- Carmeliet E. J Cardiovasc Electrophysiol. 2006;17:S2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

