Phosphoregulation of Twist1 provides a mechanism of cell fate control
- PMID: 18855684
- PMCID: PMC2744367
- DOI: 10.2174/092986708785908987
Phosphoregulation of Twist1 provides a mechanism of cell fate control
Abstract
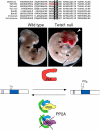
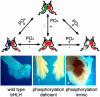
Basic Helix-loop-Helix (bHLH) factors play a significant role in both development and disease. bHLH factors function as protein dimers where two bHLH factors compose an active transcriptional complex. In various species, the bHLH factor Twist has been shown to play critical roles in diverse developmental systems such as mesoderm formation, neurogenesis, myogenesis, and neural crest cell migration and differentiation. Pathologically, Twist1 is a master regulator of epithelial-to-mesenchymal transition (EMT) and is causative of the autosomal-dominant human disease Saethre Chotzen Syndrome (SCS). Given the wide spectrum of Twist1 expression in the developing embryo and the diverse roles it plays within these forming tissues, the question of how Twist1 fills some of these specific roles has been largely unanswered. Recent work has shown that Twist's biological function can be regulated by its partner choice within a given cell. Our work has identified a phosphoregulatory circuit where phosphorylation of key residues within the bHLH domain alters partner affinities for Twist1; and more recently, we show that the DNA binding affinity of the complexes that do form is affected in a cis-element dependent manner. Such perturbations are complex as they not only affect direct transcriptional programs of Twist1, but they indirectly affect the transcriptional outcomes of any bHLH factor that can dimerize with Twist1. Thus, the resulting lineage-restricted cell fate defects are a combination of loss-of-function and gain-of-function events. Relating the observed phenotypes of defective Twist function with this complex regulatory mechanism will add insight into our understanding of the critical functions of this complex transcription factor.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

