Extended cognitive behavior therapy for cigarette smoking cessation
- PMID: 18855829
- PMCID: PMC4119230
- DOI: 10.1111/j.1360-0443.2008.02273.x
Extended cognitive behavior therapy for cigarette smoking cessation
Abstract
PRIMARY AIM: Examine the effectiveness of extended cognitive behavior therapy (CBT) in promoting longer-term smoking abstinence.
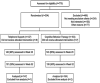
Design: Open-label treatment phase followed by extended treatment phase. Randomization conducted prior to entry into open-label treatment phase; analysis based on intention-to-treat to avoid threat of selection bias.
Setting: Community smoking cessation clinic.
Participants: A total of 304 adult smokers (> or = 18 years of age; > or = 10 cigarettes/day).
Intervention: Open-label (8 weeks): all participants received bupropion SR, nicotine patch, CBT. Extended treatment (12 weeks): participants received either CBT + voicemail monitoring and telephone counseling or telephone-based general support.
Measurements: Seven-day point prevalence abstinence, expired-air carbon monoxide.
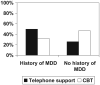
Results: At week 20 follow-up, CBT produced a higher 7-day point prevalence abstinence rate: 45% versus 29%, P = 0.006; at 52 weeks the difference in abstinence rates (31% versus 27%) was not significant. History of depression was a moderator of treatment. Those with a positive history had a better treatment response at 20 weeks when assigned to the less intensive telephone support therapy (P < 0.05).
Conclusion: The superiority of CBT to 20 weeks suggests that continued emphasis on the development of cognitive and behavioral strategies for maintaining non-smoking during an extended treatment phase may help smokers to maintain abstinence in the longer term. At present, the minimum duration of therapy is unknown.
Conflict of interest statement
Figures
References
-
- Ockene JK, Emmons KM, Mermelstein RJ, Perkins KA, Bonollo DS, Voorhees CC et al. Relapse and maintenance issues for smoking cessation. Health Psychol. 2000;19:17–31. - PubMed
-
- Silagy C, Mant D, Fowler G. Meta-analysis on efficacy of nicotine replacement therapies in smoking cessation. Lancet. 1994;343:139–42. - PubMed
-
- Fiore MC, Smith SS, Jorenby DE, Baker TB. The effectiveness of the nicotine patch for smoking cessation. JAMA. 1994;271:1940–7. - PubMed
-
- O'Brien CP, McLellan AT. Myths about the treatment of addiction. Lancet. 1996;347:237–40. - PubMed
-
- Tonnesen P, Paoletti P, Gustavsson G, Russell MA, Saracci R, Gulsvik A. Higher dose nicotine patches increase one-year smoking cessation rates: results from the European CEASE trial. Eur Respir J. 1999;13:238–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials