The EWSR1/NR4A3 fusion protein of extraskeletal myxoid chondrosarcoma activates the PPARG nuclear receptor gene
- PMID: 18855877
- PMCID: PMC4429309
- DOI: 10.1002/path.2445
The EWSR1/NR4A3 fusion protein of extraskeletal myxoid chondrosarcoma activates the PPARG nuclear receptor gene
Abstract
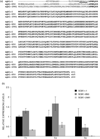
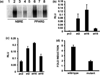
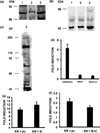
The NR4A3 nuclear receptor is implicated in the development of extraskeletal myxoid chondrosarcoma (EMC), primitive sarcoma unrelated to conventional chondrosarcomas, through a specific fusion with EWSR1 resulting in an aberrant fusion protein that is thought to disrupt the transcriptional regulation of specific target genes. We performed an expression microarray analysis of EMC tumours expressing the EWSR1/NR4A3 fusion protein, comparing their expression profiles to those of other sarcoma types. We thereby identified a set of genes significantly overexpressed in EMC relative to other sarcomas, including PPARG and NDRG2. Western blot or immunohistochemical analyses confirm that PPARG and NDRG2 are expressed in tumours positive for EWSR1/NR4A3. Bioinformatic analysis identified a DNA response element for EWSR1/NR4A3 in the PPARG promoter, and band-shift experiments and transient transfections indicate that EWSR1/NR4A3 can activate transcription through this element. Western blots further show that an isoform of the native NR4A3 receptor lacking the C-terminal domain is very highly expressed in tumours positive for EWSR1/NR4A3, and co-transfections of this isoform along with EWSR1/NR4A3 indicate that it may negatively regulate the activity of the fusion protein on the PPARG promoter. These results suggest that the overall expression of PPARG in EMC may be regulated in part by the balance between EWSR1/NR4A3 and NR4A3, and that PPARG may play a crucial role in the development of these tumours. The specific up-regulation of PPARG by EWSR1/NR4A3 may also have potential therapeutic implications.
Conflict of interest statement
There are no conflicts of interest for any of the authors
Figures

References
-
- Ohkura N, Hijikuro M, Yamamoto A, Miki K. Molecular cloning of a novel thyroid/steroid receptor superfamily gene from cultured rat neuronal cells. Biochemical and biophysical research communications. 1994;205:1959–1965. - PubMed
-
- Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988;1:183–188. - PubMed
-
- Scearce LM, Laz TM, Hazel TG, Lau LF, Taub R. RNR-1, a nuclear receptor in the NGFI-B/Nur77 family that is rapidly induced in regenerating liver. The Journal of biological chemistry. 1993;268:8855–8861. - PubMed
-
- Hedvat CV, Irving SG. The isolation and characterization of MINOR, a novel mitogen-inducible nuclear orphan receptor. Molecular endocrinology (Baltimore, Md. 1995;9:1692–1700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

