Therapy with bone marrow cells reduces liver alterations in mice chronically infected by Schistosoma mansoni
- PMID: 18855983
- PMCID: PMC2751894
- DOI: 10.3748/wjg.14.5842
Therapy with bone marrow cells reduces liver alterations in mice chronically infected by Schistosoma mansoni
Abstract
Aim: To investigate the potential of bone marrow mononuclear cells (BM-MCs) in the regeneration of hepatic lesions induced by Schistosoma mansoni (S.mansoni) chronic infection.
Methods: Female mice chronically infected with S.mansoni were treated with BM-MCs obtained from male green fluorescent protein (GFP) transgenic mice by intravenous or intralobular injections. Control mice received injections of saline in similar conditions. Enzyme-linked immunosorbent assay (ELISA) assay for transforming growth factor-beta (TGF-beta), polymerase chain reaction (PCR) for GFP DNA, immunofluorescence and morphometric studies were performed.
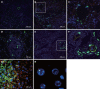
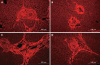
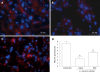
Results: Transplanted GFP(+) cells migrated to granuloma areas and reduced the percentage of liver fibrosis. The presence of donor-derived cells was confirmed by fluorescence in situ hybridization (FISH) analysis for detection of cells bearing Y chromosome and by PCR analysis for detection of GFP DNA. The levels of TGF-beta, a cytokine associated with fibrosis deposition, in liver fragments of mice submitted to therapy were reduced. The number of oval cells in liver sections of S.mansoni-infected mice increased 3-4 fold after transplantation. A partial recovery in albumin expression, which is decreased upon infection with S.mansoni, was found in livers of infected mice after cellular therapy.
Conclusion: In conclusion, transplanted BMCs migrate to and reduce the damage of chronic fibrotic liver lesions caused by S.mansoni.
Figures




Similar articles
-
Ameliorative effect of bone marrow-derived stem cells on injured liver of mice infected with Schistosoma mansoni.Korean J Parasitol. 2014 Apr;52(2):151-62. doi: 10.3347/kjp.2014.52.2.151. Epub 2014 Apr 18. Korean J Parasitol. 2014. PMID: 24850958 Free PMC article.
-
Bone marrow-derived cells migrate to the liver and contribute to the generation of different cell types in chronic Schistosoma mansoni infection.Exp Parasitol. 2015 Dec;159:29-36. doi: 10.1016/j.exppara.2015.08.005. Epub 2015 Aug 19. Exp Parasitol. 2015. PMID: 26297681
-
Homing of transplanted bone marrow cells in livers of Schistosoma mansoni-infected mice.APMIS. 2010 Apr;118(4):277-87. doi: 10.1111/j.1600-0463.2010.02585.x. APMIS. 2010. PMID: 20402673
-
Autologous bone marrow cell infusion therapy for liver cirrhosis.J Gastroenterol Hepatol. 2008 Sep;23(9):1349-53. doi: 10.1111/j.1440-1746.2008.05381.x. Epub 2008 Apr 19. J Gastroenterol Hepatol. 2008. PMID: 18422964 Review.
-
Novel findings for the development of drug therapy for various liver diseases: Current state and future prospects for our liver regeneration therapy using autologous bone marrow cells for decompensated liver cirrhosis patients.J Pharmacol Sci. 2011;115(3):274-8. doi: 10.1254/jphs.10r13fm. Epub 2011 Feb 22. J Pharmacol Sci. 2011. PMID: 21350310 Review.
Cited by
-
Human umbilical cord blood mesenchymal stem cells as a potential therapy for schistosomal hepatic fibrosis: an experimental study.Pathog Glob Health. 2023 Mar;117(2):190-202. doi: 10.1080/20477724.2022.2064795. Epub 2022 Apr 18. Pathog Glob Health. 2023. PMID: 35435145 Free PMC article.
-
Complementary Effect of Capparis Spinosa L. and Silymarin With/without Praziquantel on Mice Experimentally Infected with Schistosoma Mansoni.Helminthologia. 2018 Jan 27;55(1):21-32. doi: 10.1515/helm-2017-0055. eCollection 2018 Mar. Helminthologia. 2018. PMID: 31662624 Free PMC article.
-
Ameliorative effect of bone marrow-derived stem cells on injured liver of mice infected with Schistosoma mansoni.Korean J Parasitol. 2014 Apr;52(2):151-62. doi: 10.3347/kjp.2014.52.2.151. Epub 2014 Apr 18. Korean J Parasitol. 2014. PMID: 24850958 Free PMC article.
-
Anti-inflammatory/anti-fibrotic effects of the hepatoprotective silymarin and the schistosomicide praziquantel against Schistosoma mansoni-induced liver fibrosis.Parasit Vectors. 2012 Jan 11;5:9. doi: 10.1186/1756-3305-5-9. Parasit Vectors. 2012. PMID: 22236605 Free PMC article.
-
Bone marrow progenitor cells do not contribute to liver fibrogenic cells.World J Hepatol. 2012 Oct 27;4(10):274-83. doi: 10.4254/wjh.v4.i10.274. World J Hepatol. 2012. PMID: 23293712 Free PMC article.
References
-
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. - PubMed
-
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. - PubMed
-
- Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

