Carbapenems and SHV-1 beta-lactamase form different acyl-enzyme populations in crystals and solution
- PMID: 18922024
- PMCID: PMC2656688
- DOI: 10.1021/bi800833u
Carbapenems and SHV-1 beta-lactamase form different acyl-enzyme populations in crystals and solution
Abstract
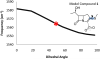
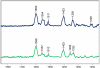
The reactions between single crystals of the SHV-1 beta-lactamase enzyme and the carbapenems, meropenem, imipenem, and ertapenem, have been studied by Raman microscopy. Aided by quantum mechanical calculations, major populations of two acyl-enzyme species, a labile Delta (2)-pyrroline and a more tightly bound Delta (1)-pyrroline, have been identified for all three compounds. These isomers differ only in the position of the double bond about the carbapenem nucleus. This discovery is consonant with X-ray crystallographic findings that also identified two populations for meropenem bound in SHV-1: one with the acyl CO group in the oxyanion hole and the second with the acyl group rotated 180 degrees compared to its expected position [Nukaga, M., Bethel, C. R., Thomson, J. M., Hujer, A. M., Distler, A. M., Anderson, V. E., Knox, J. R., and Bonomo, R. A. (2008) J. Am. Chem. Soc. (in press)]. When crystals of the Delta (1)- and Delta (2)-containing acyl-enzymes were exposed to solutions with no carbapenem, rapid deacylation of the Delta (2) species was observed by kinetic Raman experiments. However, no change in the Delta (1) population was observed over 1 h, the effective lifetime of the crystal. These observations lead to the hypothesis that the stable Delta (1) species is due to the form seen by X-ray with the acyl carbonyl outside the oxyanion hole, while the Delta (2) species corresponds to the form with the carbonyl inside the oxyanion hole. Soak-in and soak-out Raman experiments also demonstrated that tautomeric exchange between the Delta (1) and Delta (2) forms does not occur on the crystalline enzyme. When meropenem or ertapenem was reacted with SHV-1 in solution, the Raman difference spectra demonstrated that only a major population corresponding to the Delta (1) acyl-enzyme could be detected. The 1003 cm (-1) mode of the phenyl ring positioned on the C3 side chain of ertapenem acts as an effective internal Raman intensity standard, and the ratio of its intensity to that of the 1600 cm (-1) feature of Delta (1) provides an estimate of the relative populations of Delta (1). In solution, I 1600/ I 1003 equals 2, and in the crystal, I 1600 /I 1003 equals 1. This is strong evidence that the Delta (1) and Delta (2) acyl-enzymes in the crystal are present in approximately equal amounts, in agreement with the X-ray data. However, in solution there are twice as many Delta (1) species per Phe group, and this represents approximately 100% of the active sites, which is consistent with the observed inhibition of the enzyme's activity.
Figures
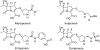







Similar articles
-
Inhibition of class A beta-lactamases by carbapenems: crystallographic observation of two conformations of meropenem in SHV-1.J Am Chem Soc. 2008 Sep 24;130(38):12656-62. doi: 10.1021/ja7111146. Epub 2008 Aug 30. J Am Chem Soc. 2008. PMID: 18761444 Free PMC article.
-
Biochemical and structural characterization of Mycobacterium tuberculosis beta-lactamase with the carbapenems ertapenem and doripenem.Biochemistry. 2010 May 4;49(17):3766-73. doi: 10.1021/bi100232q. Biochemistry. 2010. PMID: 20353175 Free PMC article.
-
Comparative review of the carbapenems.Drugs. 2007;67(7):1027-52. doi: 10.2165/00003495-200767070-00006. Drugs. 2007. PMID: 17488146 Review.
-
Tautomer-Specific Deacylation and Ω-Loop Flexibility Explain the Carbapenem-Hydrolyzing Broad-Spectrum Activity of the KPC-2 β-Lactamase.J Am Chem Soc. 2023 Apr 5;145(13):7166-7180. doi: 10.1021/jacs.2c12123. Epub 2023 Mar 27. J Am Chem Soc. 2023. PMID: 36972204 Free PMC article.
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
Cited by
-
Antimicrobial Resistance Conferred by OXA-48 β-Lactamases: Towards a Detailed Mechanistic Understanding.Antimicrob Agents Chemother. 2021 May 18;65(6):e00184-21. doi: 10.1128/AAC.00184-21. Print 2021 May 18. Antimicrob Agents Chemother. 2021. PMID: 33753332 Free PMC article. Review.
-
Natural variants modify Klebsiella pneumoniae carbapenemase (KPC) acyl-enzyme conformational dynamics to extend antibiotic resistance.J Biol Chem. 2021 Jan-Jun;296:100126. doi: 10.1074/jbc.RA120.016461. Epub 2020 Dec 3. J Biol Chem. 2021. PMID: 33257320 Free PMC article.
-
Conformational Intermediate That Controls KPC-2 Catalysis and Beta-Lactam Drug Resistance.ACS Catal. 2018 Apr 6;8(4):2741-2747. doi: 10.1021/acscatal.7b03832. Epub 2018 Feb 21. ACS Catal. 2018. PMID: 30637173 Free PMC article.
-
The Role of Hydrophobic Nodes in the Dynamics of Class A β-Lactamases.Front Microbiol. 2021 Sep 21;12:720991. doi: 10.3389/fmicb.2021.720991. eCollection 2021. Front Microbiol. 2021. PMID: 34621251 Free PMC article.
-
The binding of antibiotics in OmpF porin.Structure. 2013 Jan 8;21(1):76-87. doi: 10.1016/j.str.2012.10.014. Epub 2012 Nov 29. Structure. 2013. PMID: 23201272 Free PMC article.
References
-
- Page MG. β-Lactamase inhibitors. Drug Resist Updat. 2000;3:109–125. - PubMed
-
- Brown S, Amyes S. OXA (beta)-lactamases in Acinetobacter: the story so far. J. Antimicrob Chemother. 2006;57:1–3. - PubMed
-
- Carey PR. Raman crystallography and other biochemical applications of Raman microscopy. Annu Rev Phys Chem. 2006;57:527–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

