Comparison of hypoxia-inducible factor-1alpha expression before and after transcatheter arterial embolization in rabbit VX2 liver tumors
- PMID: 18922400
- PMCID: PMC2613192
- DOI: 10.1016/j.jvir.2008.06.017
Comparison of hypoxia-inducible factor-1alpha expression before and after transcatheter arterial embolization in rabbit VX2 liver tumors
Abstract
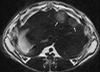
Purpose: To test the hypothesis that transcatheter arterial embolization (TAE) induces expression of hypoxia-inducible factor-1alpha (HIF-1alpha) within the same rabbit VX2 liver tumor.
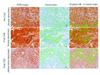
Materials and methods: Seven VX2 tumors were grown in the livers of five New Zealand white rabbits. Ultrasonography-guided biopsy was performed before and 10 minutes after TAE in all tumors. Pre- and post-TAE tumor biopsy specimens along with post-TAE whole liver tumor sections were stained with HIF-1alpha antibody and analyzed for percentage of HIF-1alpha-positive nuclei by using a spectral unmixing system mounted on a high-powered microscope. Statistical data comparisons were performed with the Wilcoxon signed-rank test (alpha = 0.05).
Results: TAE of liver tumors resulted in a statistically significant increase in the mean percentage of HIF-1alpha expression. The mean percentage of HIF-1alpha-positive stained nuclei increased from 23% +/- 3.5 in pre-TAE biopsy specimens to 41% +/- 8.7 in post-TAE biopsy specimens (P < .02). The increase was even more significant when the mean percentage of HIF-1alpha-positive stained nuclei from the same pre-TAE biopsy specimens was compared with sections from post-TAE whole tumor specimens (60% +/- 8.9, P < .02).
Conclusions: The results of this study revealed that hypoxia caused by TAE of VX2 liver tumors activates HIF-1alpha, a transcription factor that in turn regulates other pro-angiogenic factors.
Figures









References
-
- Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225–235. - PubMed
-
- Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. - PubMed
-
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. - PubMed
-
- Seki T, Tamai T, Ikeda K, et al. Rapid progression of hepatocellular carcinoma after transcatheter arterial chemoembolization and percutaneous radiofrequency ablation in the primary tumour region. Eur J Gastroenterol Hepatol. 2001;13:291–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

