Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats
- PMID: 18922775
- PMCID: PMC2575484
- DOI: 10.1073/pnas.0808488105
Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats
Abstract
Substitutive cell therapy using fetal striatal grafts has demonstrated preliminary clinical success in patients with Huntington's disease, but the logistics required for accessing fetal cells preclude its extension to the relevant population of patients. Human embryonic stem (hES) cells theoretically meet this challenge, because they can be expanded indefinitely and differentiated into any cell type. We have designed an in vitro protocol combining substrates, media, and cytokines to push hES cells along the neural lineage, up to postmitotic neurons expressing striatal markers. The therapeutic potential of such hES-derived cells was further substantiated by their in vivo differentiation into striatal neurons following xenotransplantation into adult rats. Our results open the way toward hES cell therapy for Huntington's disease. Long-term proliferation of human neural progenitors leads, however, to xenograft overgrowth in the rat brain, suggesting that the path to the clinic requires a way to switch them off after grafting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
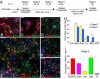
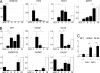
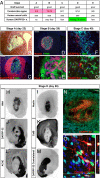
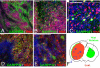
References
-
- Peschanski M, Bachoud-Levi AC, Hantraye P. Integrating fetal neural transplants into a therapeutic strategy: The example of Huntington's disease. Brain. 2004;127:1219–1228. - PubMed
-
- Bachoud-Levi AC, et al. Effect of fetal neural transplants in patients with Huntington's disease 6 years after surgery: A long-term follow-up study. Lancet Neurol. 2006;5:303–309. - PubMed
-
- Bachoud-Levi AC, et al. Motor and cognitive improvements in patients with Huntington's disease after neural transplantation. Lancet. 2000;356:1975–1979. - PubMed
-
- Gaura V, et al. Striatal neural grafting improves cortical metabolism in Huntington's disease patients. Brain. 2004;127:65–72. - PubMed
-
- Peschanski M, Dunnett SB. Cell therapy for Huntington's disease, the next step forward. Lancet Neurol. 2002;1:81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

