Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1
- PMID: 18922801
- PMCID: PMC2602882
- DOI: 10.1074/jbc.M805507200
Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1
Abstract
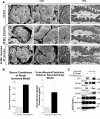
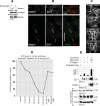
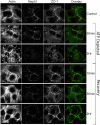
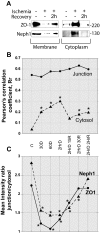
Glomerular injury is often characterized by the effacement of podocytes, loss of slit diaphragms, and proteinuria. Renal ischemia or the loss of blood flow to the kidneys has been widely associated with tubular and endothelial injury but rarely has been shown to induce podocyte damage and disruption of the slit diaphragm. In this study, we have used an in vivo rat ischemic model to demonstrate that renal ischemia induces podocyte effacement with loss of slit diaphragm and proteinuria. Biochemical analysis of the ischemic glomerulus shows that ischemia induces rapid loss of interaction between slit diaphragm junctional proteins Neph1 and ZO-1. To further understand the effect of ischemia on molecular interactions between slit diaphragm proteins, a cell culture model was employed to study the binding between Neph1 and ZO-1. Under physiologic conditions, Neph1 co-localized with ZO-1 at cell-cell contacts in cultured human podocytes. Induction of injury by ATP depletion resulted in rapid loss of Neph1 and ZO-1 binding and redistribution of Neph1 and ZO-1 proteins from cell membrane to the cytoplasm. Recovery resulted in increased Neph1 tyrosine phosphorylation, restoring Neph1 and ZO-1 binding and their localization at the cell membrane. We further demonstrate that tyrosine phosphorylation of Neph1 mediated by Fyn results in significantly increased Neph1 and ZO-1 binding, suggesting a critical role for Neph1 tyrosine phosphorylation in reorganizing the Neph1-ZO-1 complex. This study documents that renal ischemia induces dynamic changes in the molecular interactions between slit diaphragm proteins, leading to podocyte damage and proteinuria.
Figures


References
-
- Molitoris, B. A., Melnikov, V. Y., Okusa, M. D., and Himmelfarb, J. (2008) Nat. Clin. Pract. Nephrol. 4 154-165 - PubMed
-
- Molitoris, B. A., and Sutton, T. A. (2004) Kidney Int. 66 496-499 - PubMed
-
- Kelly, K. J., and Molitoris, B. A. (2000) Semin Nephrol. 20 4-19 - PubMed
-
- Molitoris, B. A. (2003) J. Am. Soc. Nephrol. 14 265-267 - PubMed
-
- Parikh, C. R., Edelstein, C. L., Devarajan, P., and Cantley, L. (2007) J. Investig. Med. 55 333-340 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

