BALB/Mtv-null mice responding to strong mouse mammary tumor virus superantigens restrict mammary tumorigenesis
- PMID: 18922863
- PMCID: PMC2612332
- DOI: 10.1128/JVI.01374-08
BALB/Mtv-null mice responding to strong mouse mammary tumor virus superantigens restrict mammary tumorigenesis
Abstract
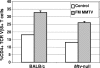
The absence of endogenous mouse mammary tumor viruses (MMTVs) in the congenic mouse strain, BALB/Mtv-null, restricts the early steps of exogenous C3H MMTV infection, preventing the superantigen (Sag) response and mammary tumorigenesis. Here we demonstrate that BALB/Mtv-null mice also resist tumor induction by FM MMTV, which encodes a stronger Sag compared to C3H MMTV. In contrast to infections with C3H MMTV, Mtv-null mice show FM-MMTV Sag-specific responses comparable to those observed in susceptible BALB/c mice. Neither virus shows significant replication in the spleen or mammary gland. Thus, Mtv-null mice restrict MMTV replication and mammary tumorigenesis even after a robust Sag response.
Figures


References
-
- Acha-Orbea, H., A. N. Shakhov, L. Scarpellino, E. Kolb, V. Muller, A. Vessaz-Shaw, R. Fuchs, K. Blochlinger, P. Rollini, J. Billotte, M. Sarafidou, H. R. MacDonald, and H. Diggelmann. 1991. Clonal deletion of V β 14-bearing T cells in mice transgenic for mammary tumour virus. Nature 350207-211. - PubMed
-
- Beutner, U., B. McLellan, E. Kraus, and B. T. Huber. 1996. Lack of MMTV superantigen presentation in MHC class II-deficient mice. Cell. Immunol. 168141-147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

