Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies
- PMID: 18922865
- PMCID: PMC2612342
- DOI: 10.1128/JVI.01583-08
Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies
Abstract
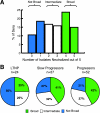
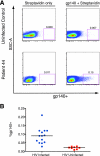
Induction of broadly cross-reactive neutralizing antibodies (NAb) is an important goal for a prophylactic human immunodeficiency virus type 1 (HIV-1) vaccine. Some HIV-infected patients make a NAb response that reacts with diverse strains of HIV-1, but most candidate vaccines have induced NAb only against a subset of highly sensitive isolates. To better understand the nature of broad NAb responses that arise during natural infection, we screened patients for sera able to neutralize diverse HIV strains and explored the frequency and phenotype of their peripheral Envelope-specific B cells. We screened 113 HIV-infected patients of various clinical statuses for the prevalence of broad NAb. Sera able to neutralize at least four of five viral isolates were found in over one-third of progressors and slow progressors, but much less frequently in aviremic long-term nonprogressors. Most Env-specific antibody-secreting B cells were CD27(hi) CD38(hi) plasmablasts, and the total plasmablast frequency was higher in HIV-infected patients than in uninfected donors. We found that 0.0031% of B cells and 0.047% of plasmablasts secreted Env-specific immunoglobulin G (IgG) in an enzyme-linked immunospot (ELISPOT) assay. We developed a novel staining protocol to label HIV-specific B cells with Env gp140 protein. A total of 0.09% of B cells were found to be Env-specific by this method, a frequency far higher than that indicated by ELISPOT assay. gp140-labeled B cells were predominantly CD27(+) and surface IgG(+). These data describe the breadth and titer of serum NAb and the frequency and phenotype of HIV-specific B cells in a cohort of patients with broad cross-neutralizing antibody responses that are potential goals for vaccines for HIV.
Figures




References
-
- Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science 27254-60. - PubMed
-
- Albert, J., B. Abrahamsson, K. Nagy, E. Aurelius, H. Gaines, G. Nystrom, and E. M. Fenyo. 1990. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS 4107-112. - PubMed
-
- Anderson, S. M., M. M. Tomayko, and M. J. Shlomchik. 2006. Intrinsic properties of human and murine memory B cells. Immunol. Rev. 211280-294. - PubMed
-
- Arendrup, M., C. Nielsen, J. E. Hansen, C. Pedersen, L. Mathiesen, and J. O. Nielsen. 1992. Autologous HIV-1 neutralizing antibodies: emergence of neutralization-resistant escape virus and subsequent development of escape virus neutralizing antibodies. J. Acquir. Immune Defic. Syndr. 5303-307. - PubMed
-
- Arthos, J., C. Cicala, E. Martinelli, K. Macleod, D. Van Ryk, D. Wei, Z. Xiao, T. D. Veenstra, T. P. Conrad, R. A. Lempicki, S. McLaughlin, M. Pascuccio, R. Gopaul, J. McNally, C. C. Cruz, N. Censoplano, E. Chung, K. N. Reitano, S. Kottilil, D. J. Goode, and A. S. Fauci. 2008. HIV-1 envelope protein binds to and signals through integrin α4β7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 9301-309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

