FGFR3 promotes synchondrosis closure and fusion of ossification centers through the MAPK pathway
- PMID: 18923003
- PMCID: PMC2638772
- DOI: 10.1093/hmg/ddn339
FGFR3 promotes synchondrosis closure and fusion of ossification centers through the MAPK pathway
Abstract
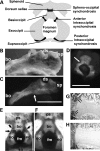
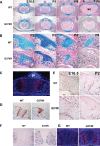
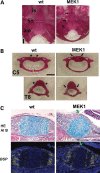
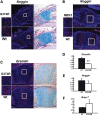
Activating mutations in FGFR3 cause achondroplasia and thanatophoric dysplasia, the most common human skeletal dysplasias. In these disorders, spinal canal and foramen magnum stenosis can cause serious neurologic complications. Here, we provide evidence that FGFR3 and MAPK signaling in chondrocytes promote synchondrosis closure and fusion of ossification centers. We observed premature synchondrosis closure in the spine and cranial base in human cases of homozygous achondroplasia and thanatophoric dysplasia as well as in mouse models of achondroplasia. In both species, premature synchondrosis closure was associated with increased bone formation. Chondrocyte-specific activation of Fgfr3 in mice induced premature synchondrosis closure and enhanced osteoblast differentiation around synchondroses. FGF signaling in chondrocytes increases Bmp ligand mRNA expression and decreases Bmp antagonist mRNA expression in a MAPK-dependent manner, suggesting a role for Bmp signaling in the increased bone formation. The enhanced bone formation would accelerate the fusion of ossification centers and limit the endochondral bone growth. Spinal canal and foramen magnum stenosis in heterozygous achondroplasia patients, therefore, may occur through premature synchondrosis closure. If this is the case, then any growth-promoting treatment for these complications of achondroplasia must precede the timing of the synchondrosis closure.
Figures




Similar articles
-
Genetic inactivation of ERK1 and ERK2 in chondrocytes promotes bone growth and enlarges the spinal canal.J Orthop Res. 2011 Mar;29(3):375-9. doi: 10.1002/jor.21262. Epub 2010 Oct 4. J Orthop Res. 2011. PMID: 20922792 Free PMC article.
-
Constitutive activation of MEK1 in chondrocytes causes Stat1-independent achondroplasia-like dwarfism and rescues the Fgfr3-deficient mouse phenotype.Genes Dev. 2004 Feb 1;18(3):290-305. doi: 10.1101/gad.1179104. Genes Dev. 2004. PMID: 14871928 Free PMC article.
-
HDAC6 deficiency or inhibition blocks FGFR3 accumulation and improves bone growth in a model of achondroplasia.Hum Mol Genet. 2016 Oct 1;25(19):4227-4243. doi: 10.1093/hmg/ddw255. Epub 2016 Aug 9. Hum Mol Genet. 2016. PMID: 27506979
-
Achondroplasia: Development, pathogenesis, and therapy.Dev Dyn. 2017 Apr;246(4):291-309. doi: 10.1002/dvdy.24479. Epub 2017 Mar 2. Dev Dyn. 2017. PMID: 27987249 Free PMC article. Review.
-
[Molecular basis of achondroplasia, hypochondroplasia, and thanatophoric dysplasia].Chir Narzadow Ruchu Ortop Pol. 2000;65(3):327-33. Chir Narzadow Ruchu Ortop Pol. 2000. PMID: 11057021 Review. Polish.
Cited by
-
Meckel's and condylar cartilages anomalies in achondroplasia result in defective development and growth of the mandible.Hum Mol Genet. 2016 Jul 15;25(14):2997-3010. doi: 10.1093/hmg/ddw153. Epub 2016 Jun 3. Hum Mol Genet. 2016. PMID: 27260401 Free PMC article.
-
Comparative transcriptome analysis provides insight into the molecular targets and signaling pathways of deer TGF-1 regulating chondrocytes proliferation and differentiation.Mol Biol Rep. 2023 Apr;50(4):3155-3166. doi: 10.1007/s11033-023-08265-z. Epub 2023 Jan 25. Mol Biol Rep. 2023. PMID: 36696024
-
Thoracolumbar stenosis and neurologic symptoms: Quantitative MRI in achondroplasia.J Neuroimaging. 2022 Sep;32(5):884-893. doi: 10.1111/jon.13015. Epub 2022 Jun 12. J Neuroimaging. 2022. PMID: 35691933 Free PMC article.
-
FGFR3 induces degradation of BMP type I receptor to regulate skeletal development.Biochim Biophys Acta. 2014 Jul;1843(7):1237-47. doi: 10.1016/j.bbamcr.2014.03.011. Epub 2014 Mar 20. Biochim Biophys Acta. 2014. PMID: 24657641 Free PMC article.
-
FGFR antagonists restore defective mandibular bone repair in a mouse model of osteochondrodysplasia.Bone Res. 2025 Jan 21;13(1):12. doi: 10.1038/s41413-024-00385-x. Bone Res. 2025. PMID: 39837840 Free PMC article.
References
-
- van der Eerden B.C., Karperien M., Wit J.M. Systemic and local regulation of the growth plate. Endocr. Rev. 2003;24:782–801. - PubMed
-
- Nilsson O., Marino R., De Luca F., Phillip M., Baron J. Endocrine regulation of the growth plate. Horm. Res. 2005;64:157–165. - PubMed
-
- Peters K., Ornitz D., Werner S., Williams L. Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev. Biol. 1993;155:423–430. - PubMed
-
- Delezoide A.L., Benoist-Lasselin C., Legeai-Mallet L., Le Merrer M., Munnich A., Vekemans M., Bonaventure J. Spatio-temporal expression of FGFR 1, 2 and 3 genes during human embryo-fetal ossification. Mech. Dev. 1998;77:19–30. - PubMed
-
- Rousseau F., Bonaventure J., Legeai-Mallet L., Pelet A., Rozet J.M., Maroteaux P., Le Merrer M., Munnich A. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994;371:252–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

