Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum
- PMID: 18923023
- PMCID: PMC2814340
- DOI: 10.1523/JNEUROSCI.2518-08.2008
Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum
Abstract
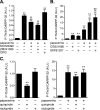
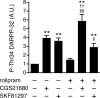
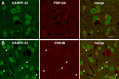
Phosphodiesterase (PDE) is a critical regulator of cAMP/protein kinase A (PKA) signaling in cells. Multiple PDEs with different substrate specificities and subcellular localization are expressed in neurons. Dopamine plays a central role in the regulation of motor and cognitive functions. The effect of dopamine is largely mediated through the cAMP/PKA signaling cascade, and therefore controlled by PDE activity. We used in vitro and in vivo biochemical techniques to dissect the roles of PDE4 and PDE10A in dopaminergic neurotransmission in mouse striatum by monitoring the ability of selective PDE inhibitors to regulate phosphorylation of presynaptic [e.g., tyrosine hydroxylase (TH)] and postsynaptic [e.g., dopamine- and cAMP-regulated phosphoprotein of M(r) 32 kDa (DARPP-32)] PKA substrates. The PDE4 inhibitor, rolipram, induced a large increase in TH Ser40 phosphorylation at dopaminergic terminals that was associated with a commensurate increase in dopamine synthesis and turnover in striatum in vivo. Rolipram induced a small increase in DARPP-32 Thr34 phosphorylation preferentially in striatopallidal neurons by activating adenosine A(2A) receptor signaling in striatum. In contrast, the PDE10A inhibitor, papaverine, had no effect on TH phosphorylation or dopamine turnover, but instead robustly increased DARPP-32 Thr34 and GluR1 Ser845 phosphorylation in striatal neurons. Inhibition of PDE10A by papaverine activated cAMP/PKA signaling in both striatonigral and striatopallidal neurons, resulting in potentiation of dopamine D(1) receptor signaling and inhibition of dopamine D(2) receptor signaling. These biochemical results are supported by immunohistochemical data demonstrating differential localization of PDE10A and PDE4 in striatum. These data underscore the importance of individual brain-enriched cyclic-nucleotide PDE isoforms as therapeutic targets for neuropsychiatric and neurodegenerative disorders affecting dopamine neurotransmission.
Figures






References
-
- Becker A, Grecksch G. Phosphodiesterase inhibitors–are they potential neuroleptic drugs? Behav Brain Res. 2008;186:155–160. - PubMed
-
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. - PubMed
-
- Boyar WC, Altar CA. Modulation of in vivo dopamine release by D2 but not D1 receptor agonists and antagonists. J Neurochem. 1987;48:824–831. - PubMed
-
- Cherry JA, Davis RL. Cyclic AMP phosphodiesterases are localized in regions of the mouse brain associated with reinforcement, movement, and affect. J Comp Neurol. 1999;407:287–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
