Supraspinal glial-neuronal interactions contribute to descending pain facilitation
- PMID: 18923025
- PMCID: PMC2660868
- DOI: 10.1523/JNEUROSCI.3593-08.2008
Supraspinal glial-neuronal interactions contribute to descending pain facilitation
Abstract
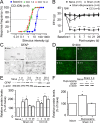
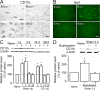
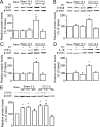
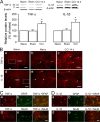
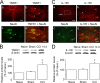
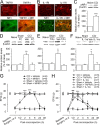
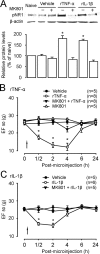
Spinal glial reaction and proinflammatory cytokine induction play an important role in the development of chronic pain states after tissue and nerve injury. The present study investigated the cellular and molecular mechanisms underlying descending facilitation of neuropathic pain with an emphasis on supraspinal glial-neuronal relationships. An early and transient reaction of microglia and prolonged reaction of astrocytes were found after chronic constriction injury (CCI) of the rat infraorbital nerve in the rostral ventromedial medulla (RVM), a major component of brainstem descending pain modulatory circuitry. There were prolonged elevations of cytokines tumor necrosis factor-alpha (TNF-alpha) and interleukin-1beta (IL-1beta) after CCI, and they were expressed in RVM astrocytes at 14 d after injury. Intra-RVM injection of microglial and astrocytic inhibitors attenuated mechanical hyperalgesia and allodynia at 3 and 14 d after CCI, respectively. Moreover, TNFR1 and IL-1R, receptors for TNF-alpha and IL-1beta, respectively, were expressed primarily in RVM neurons exhibiting immunoreactivity to the NMDA receptor (NMDAR) subunit NR1. CCI increased TNFR1 and IL-1R levels and NR1 phosphorylation in the RVM. Neutralization of endogenous TNF-alpha and IL-1beta in the RVM significantly reduced CCI-induced behavioral hypersensitivity and attenuated NR1 phosphorylation. Finally, intra-RVM administration of recombinant TNF-alpha or IL-1beta upregulated NR1 phosphorylation and caused a reversible and NMDAR-dependent allodynia in normal rats, further suggesting that TNF-alpha and IL-1beta couple glial hyperactivation with NMDAR function. These studies have addressed a novel contribution of supraspinal astrocytes and associated cytokines as well as central glial-neuronal interactions to the enhancement of descending facilitation of neuropathic pain.
Figures

Similar articles
-
Spinal astrocytic activation contributes to mechanical allodynia in a rat model of cyclophosphamide-induced cystitis.Mol Pain. 2016 Nov 15;12:1744806916674479. doi: 10.1177/1744806916674479. Print 2016. Mol Pain. 2016. PMID: 27852964 Free PMC article.
-
Spinal 5-HT(3) receptor activation induces behavioral hypersensitivity via a neuronal-glial-neuronal signaling cascade.J Neurosci. 2011 Sep 7;31(36):12823-36. doi: 10.1523/JNEUROSCI.1564-11.2011. J Neurosci. 2011. Retraction in: J Neurosci. 2012 Dec 12;32(50):18270. doi: 10.1523/JNEUROSCI.5032-12.2012. PMID: 21900561 Free PMC article. Retracted.
-
Chemokine signaling involving chemokine (C-C motif) ligand 2 plays a role in descending pain facilitation.Neurosci Bull. 2012 Apr;28(2):193-207. doi: 10.1007/s12264-012-1218-6. Neurosci Bull. 2012. PMID: 22466130 Free PMC article.
-
Role of interleukin-1beta during pain and inflammation.Brain Res Rev. 2009 Apr;60(1):57-64. doi: 10.1016/j.brainresrev.2008.12.020. Epub 2008 Dec 31. Brain Res Rev. 2009. PMID: 19166877 Free PMC article. Review.
-
Neuron-glia crosstalk gets serious: role in pain hypersensitivity.Curr Opin Anaesthesiol. 2008 Oct;21(5):570-9. doi: 10.1097/ACO.0b013e32830edbdf. Curr Opin Anaesthesiol. 2008. PMID: 18784481 Free PMC article. Review.
Cited by
-
Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: the effects of interleukin-10 and glial inhibitors.Mol Pain. 2009 Dec 21;5:75. doi: 10.1186/1744-8069-5-75. Mol Pain. 2009. PMID: 20025765 Free PMC article.
-
Central mechanisms of pathological pain.Nat Med. 2010 Nov;16(11):1258-66. doi: 10.1038/nm.2231. Epub 2010 Oct 14. Nat Med. 2010. PMID: 20948531 Review.
-
MrgC agonism at central terminals of primary sensory neurons inhibits neuropathic pain.Pain. 2014 Mar;155(3):534-544. doi: 10.1016/j.pain.2013.12.008. Epub 2013 Dec 11. Pain. 2014. PMID: 24333779 Free PMC article.
-
A neuron-to-astrocyte Wnt5a signal governs astrogliosis during HIV-associated pain pathogenesis.Brain. 2022 Nov 21;145(11):4108-4123. doi: 10.1093/brain/awac015. Brain. 2022. PMID: 35040478 Free PMC article.
-
Necrostatin-1 Ameliorates Peripheral Nerve Injury-Induced Neuropathic Pain by Inhibiting the RIP1/RIP3 Pathway.Front Cell Neurosci. 2019 May 15;13:211. doi: 10.3389/fncel.2019.00211. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31156396 Free PMC article.
References
-
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. - PubMed
-
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces abnormal pain sensation like those seen in man. Pain. 1988;33:87–107. - PubMed
-
- Bursztajn S, Rutkowski MD, Deleo JA. The role of the N-methyl-d-aspartate receptor NR1 subunit in peripheral nerve injury-induced mechanical allodynia, glial activation and chemokine expression in the mouse. Neuroscience. 2004;125:269–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
