RMI, a new OB-fold complex essential for Bloom syndrome protein to maintain genome stability
- PMID: 18923082
- PMCID: PMC2569887
- DOI: 10.1101/gad.1708608
RMI, a new OB-fold complex essential for Bloom syndrome protein to maintain genome stability
Abstract
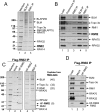
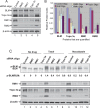
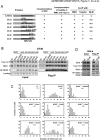
BLM, the helicase mutated in Bloom syndrome, associates with topoisomerase 3alpha, RMI1 (RecQ-mediated genome instability), and RPA, to form a complex essential for the maintenance of genome stability. Here we report a novel component of the BLM complex, RMI2, which interacts with RMI1 through two oligonucleotide-binding (OB)-fold domains similar to those in RPA. The resulting complex, named RMI, differs from RPA in that it lacks obvious DNA-binding activity. Nevertheless, RMI stimulates the dissolution of a homologous recombination intermediate in vitro and is essential for the stability, localization, and function of the BLM complex in vivo. Notably, inactivation of RMI2 in chicken DT40 cells results in an increased level of sister chromatid exchange (SCE)--the hallmark feature of Bloom syndrome cells. Epistasis analysis revealed that RMI2 and BLM suppress SCE within the same pathway. A point mutation in the OB domain of RMI2 disrupts the association between BLM and the rest of the complex, and abrogates the ability of RMI2 to suppress elevated SCE. Our data suggest that multi-OB-fold complexes mediate two modes of BLM action: via RPA-mediated protein-DNA interaction, and via RMI-mediated protein-protein interactions.
Figures




Comment in
-
More complexity to the Bloom's syndrome complex.Genes Dev. 2008 Oct 15;22(20):2737-42. doi: 10.1101/gad.1732808. Genes Dev. 2008. PMID: 18923071 Free PMC article.
References
-
- Bachrati C.Z., Hickson I.D. RecQ helicases: Guardian angels of the DNA replication fork. Chromosoma. 2008;117:219–233. - PubMed
-
- Bochkareva E., Korolev S., Bochkarev A. The role for zinc in replication protein A. J. Biol. Chem. 2000;275:27332–27338. - PubMed
-
- Briggs G.S., Mahdi A.A., Wen Q., Lloyd R.G. DNA binding by the substrate specificity (wedge) domain of RecG helicase suggests a role in processivity. J. Biol. Chem. 2005;280:13921–13927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
