Diffusion in brain extracellular space
- PMID: 18923183
- PMCID: PMC2785730
- DOI: 10.1152/physrev.00027.2007
Diffusion in brain extracellular space
Abstract
Diffusion in the extracellular space (ECS) of the brain is constrained by the volume fraction and the tortuosity and a modified diffusion equation represents the transport behavior of many molecules in the brain. Deviations from the equation reveal loss of molecules across the blood-brain barrier, through cellular uptake, binding, or other mechanisms. Early diffusion measurements used radiolabeled sucrose and other tracers. Presently, the real-time iontophoresis (RTI) method is employed for small ions and the integrative optical imaging (IOI) method for fluorescent macromolecules, including dextrans or proteins. Theoretical models and simulations of the ECS have explored the influence of ECS geometry, effects of dead-space microdomains, extracellular matrix, and interaction of macromolecules with ECS channels. Extensive experimental studies with the RTI method employing the cation tetramethylammonium (TMA) in normal brain tissue show that the volume fraction of the ECS typically is approximately 20% and the tortuosity is approximately 1.6 (i.e., free diffusion coefficient of TMA is reduced by 2.6), although there are regional variations. These parameters change during development and aging. Diffusion properties have been characterized in several interventions, including brain stimulation, osmotic challenge, and knockout of extracellular matrix components. Measurements have also been made during ischemia, in models of Alzheimer's and Parkinson's diseases, and in human gliomas. Overall, these studies improve our conception of ECS structure and the roles of glia and extracellular matrix in modulating the ECS microenvironment. Knowledge of ECS diffusion properties is valuable in contexts ranging from understanding extrasynaptic volume transmission to the development of paradigms for drug delivery to the brain.
Figures
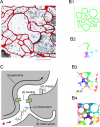








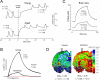
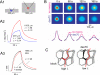


References
-
- Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45:545–552. - PubMed
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Agnati LF, Fuxe K, Nicholson C, Syková E. Volume Transmission Revisited. Elsevier; Amsterdam: 2000.
-
- Agnati LF, Leo G, Zanardi A, Genedani S, Rivera A, Fuxe K, Guidolin D. Volume transmission and wiring transmission from cellular to molecular networks: history and perspectives. Acta Physiol (Oxf) 2006;187:329–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

