Combining results from lectin affinity chromatography and glycocapture approaches substantially improves the coverage of the glycoproteome
- PMID: 18923192
- PMCID: PMC2634582
- DOI: 10.1074/mcp.M800272-MCP200
Combining results from lectin affinity chromatography and glycocapture approaches substantially improves the coverage of the glycoproteome
Abstract
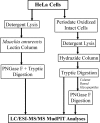
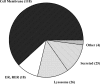
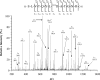
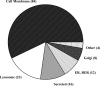
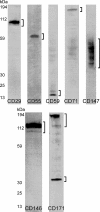
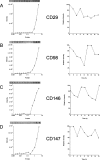
Identification of glycosylated proteins, especially those in the plasma membrane, has the potential of defining diagnostic biomarkers and therapeutic targets as well as increasing our understanding of changes occurring in the glycoproteome during normal differentiation and disease processes. Although many cellular proteins are glycosylated they are rarely identified by mass spectrometric analysis (e.g. shotgun proteomics) of total cell lysates. Therefore, methods that specifically target glycoproteins are necessary to facilitate their isolation from total cell lysates prior to their identification by mass spectrometry-based analysis. To enrich for plasma membrane glycoproteins the methods must selectively target characteristics associated with proteins within this compartment. We demonstrate that the application of two methods, one that uses periodate to label glycoproteins of intact cells and a hydrazide resin to capture the labeled glycoproteins and another that targets glycoproteins with sialic acid residues using lectin affinity chromatography, in conjunction with liquid chromatography-tandem mass spectrometry is effective for plasma membrane glycoprotein identification. We demonstrate that this combination of methods dramatically increases coverage of the plasma membrane proteome (more than one-half of the membrane glycoproteins were identified by the two methods uniquely) and also results in the identification of a large number of secreted glycoproteins. Our approach avoids the need for subcellular fractionation and utilizes a simple detergent lysis step that effectively solubilizes membrane glycoproteins. The plasma membrane localization of a subset of proteins identified was validated, and the dynamics of their expression in HeLa cells was evaluated during the cell cycle. Results obtained from the cell cycle studies demonstrate that plasma membrane protein expression can change up to 4-fold as cells transit the cell cycle and demonstrate the need to consider such changes when carrying out quantitative proteomics comparison of cell lines.
Figures
Similar articles
-
Cell surface and secreted protein profiles of human thyroid cancer cell lines reveal distinct glycoprotein patterns.J Proteome Res. 2009 Aug;8(8):3958-68. doi: 10.1021/pr900278c. J Proteome Res. 2009. PMID: 19530676 Free PMC article.
-
Human plasma N-glycoproteome analysis by immunoaffinity subtraction, hydrazide chemistry, and mass spectrometry.J Proteome Res. 2005 Nov-Dec;4(6):2070-80. doi: 10.1021/pr0502065. J Proteome Res. 2005. PMID: 16335952 Free PMC article.
-
An approach to quantifying N-linked glycoproteins by enzyme-catalyzed 18O3-labeling of solid-phase enriched glycopeptides.Anal Chem. 2010 Sep 15;82(18):7722-8. doi: 10.1021/ac101564t. Anal Chem. 2010. PMID: 20795641
-
Mass Spectrometry-Based Chemical and Enzymatic Methods for Global Analysis of Protein Glycosylation.Acc Chem Res. 2018 Aug 21;51(8):1796-1806. doi: 10.1021/acs.accounts.8b00200. Epub 2018 Jul 16. Acc Chem Res. 2018. PMID: 30011186 Free PMC article. Review.
-
Using lectins to harvest the plasma/serum glycoproteome.Electrophoresis. 2012 Jul;33(12):1746-54. doi: 10.1002/elps.201100567. Electrophoresis. 2012. PMID: 22740463 Review.
Cited by
-
Proteomic analysis of the extracellular matrix produced by mesenchymal stromal cells: implications for cell therapy mechanism.PLoS One. 2013 Nov 14;8(11):e79283. doi: 10.1371/journal.pone.0079283. eCollection 2013. PLoS One. 2013. PMID: 24244468 Free PMC article.
-
Challenges in plasma membrane phosphoproteomics.Expert Rev Proteomics. 2011 Aug;8(4):483-94. doi: 10.1586/epr.11.40. Expert Rev Proteomics. 2011. PMID: 21819303 Free PMC article. Review.
-
Cell surface and secreted protein profiles of human thyroid cancer cell lines reveal distinct glycoprotein patterns.J Proteome Res. 2009 Aug;8(8):3958-68. doi: 10.1021/pr900278c. J Proteome Res. 2009. PMID: 19530676 Free PMC article.
-
Cocapture of cognate and bystander antigens can activate autoreactive B cells.Proc Natl Acad Sci U S A. 2017 Jan 24;114(4):734-739. doi: 10.1073/pnas.1614472114. Epub 2017 Jan 5. Proc Natl Acad Sci U S A. 2017. PMID: 28057865 Free PMC article.
-
Functional decorations: post-translational modifications and heart disease delineated by targeted proteomics.Genome Med. 2013 Feb 28;5(2):20. doi: 10.1186/gm424. eCollection 2013. Genome Med. 2013. PMID: 23445784 Free PMC article. Review.
References
-
- Apweiler, R., Hermjakob, H., and Sharon, N. ( 1999) On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta 1473, 4–8 - PubMed
-
- Schachter, H. ( 1991) The ‘yellow brick road’ to branched complex N-glycans. Glycobiology 1, 453–461 - PubMed
-
- Trombetta, E. S., and Parodi, A. J. ( 2003) Quality control and protein folding in the secretory pathway. Annu. Rev. Cell Dev. Biol. 19, 649–676 - PubMed
-
- Lowe, J. B., and Marth, J. D. ( 2003) A genetic approach to Mammalian glycan function. Annu. Rev. Biochem. 72, 643–691 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

