Direct control of paralysed muscles by cortical neurons
- PMID: 18923392
- PMCID: PMC3159518
- DOI: 10.1038/nature07418
Direct control of paralysed muscles by cortical neurons
Abstract
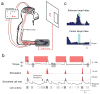
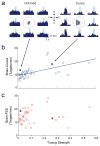
A potential treatment for paralysis resulting from spinal cord injury is to route control signals from the brain around the injury by artificial connections. Such signals could then control electrical stimulation of muscles, thereby restoring volitional movement to paralysed limbs. In previously separate experiments, activity of motor cortex neurons related to actual or imagined movements has been used to control computer cursors and robotic arms, and paralysed muscles have been activated by functional electrical stimulation. Here we show that Macaca nemestrina monkeys can directly control stimulation of muscles using the activity of neurons in the motor cortex, thereby restoring goal-directed movements to a transiently paralysed arm. Moreover, neurons could control functional stimulation equally well regardless of any previous association to movement, a finding that considerably expands the source of control signals for brain-machine interfaces. Monkeys learned to use these artificial connections from cortical cells to muscles to generate bidirectional wrist torques, and controlled multiple neuron-muscle pairs simultaneously. Such direct transforms from cortical activity to muscle stimulation could be implemented by autonomous electronic circuitry, creating a relatively natural neuroprosthesis. These results are the first demonstration that direct artificial connections between cortical cells and muscles can compensate for interrupted physiological pathways and restore volitional control of movement to paralysed limbs.
Conflict of interest statement
Figures


References
-
- Jackson A, Moritz CT, Mavoori J, Lucas TH, Fetz EE. The Neurochip BCI: towards a neural prosthesis for upper limb function. IEEE Trans Neural Syst Rehabil Eng. 2006;14:187–90. - PubMed
-
- Lauer RT, Peckham PH, Kilgore KL. EEG-based control of a hand grasp neuroprosthesis. Neuroreport. 1999;10:1767–71. - PubMed
-
- Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature. 2008 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

