Clustering of VASP actively drives processive, WH2 domain-mediated actin filament elongation
- PMID: 18923426
- PMCID: PMC2585163
- DOI: 10.1038/emboj.2008.211
Clustering of VASP actively drives processive, WH2 domain-mediated actin filament elongation
Abstract
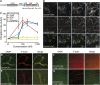
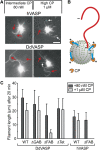
Vasodilator-stimulated phosphoprotein (VASP) is a key regulator of dynamic actin structures like filopodia and lamellipodia, but its precise function in their formation is controversial. Using in vitro TIRF microscopy, we show for the first time that both human and Dictyostelium VASP are directly involved in accelerating filament elongation by delivering monomeric actin to the growing barbed end. In solution, DdVASP markedly accelerated actin filament elongation in a concentration-dependent manner but was inhibited by low concentrations of capping protein (CP). In striking contrast, VASP clustered on functionalized beads switched to processive filament elongation that became insensitive even to very high concentrations of CP. Supplemented with the in vivo analysis of VASP mutants and an EM structure of the protein, we propose a mechanism by which membrane-associated VASP oligomers use their WH2 domains to effect both the tethering of actin filaments and their processive elongation in sites of active actin assembly.
Figures





References
-
- Aebi U, Baschong W (2006) Glycerol spraying/low-angle rotary metal shadowing. In Cell Biology—A Laboratory Handbook, J Celis (ed), 3rd ed, pp 241–246. London: Elsevier
-
- Bachmann C, Fischer L, Walter U, Reinhard M (1999) The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J Biol Chem 274: 23549–23557 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

