A role for the two-helix finger of the SecA ATPase in protein translocation
- PMID: 18923526
- PMCID: PMC4354775
- DOI: 10.1038/nature07439
A role for the two-helix finger of the SecA ATPase in protein translocation
Abstract
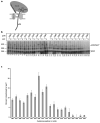
An important step in the biosynthesis of many proteins is their partial or complete translocation across the plasma membrane in prokaryotes or the endoplasmic reticulum membrane in eukaryotes. In bacteria, secretory proteins are generally translocated after completion of their synthesis by the interaction of the cytoplasmic ATPase SecA and a protein-conducting channel formed by the SecY complex. How SecA moves substrates through the SecY channel is unclear. However, a recent structure of a SecA-SecY complex raises the possibility that the polypeptide chain is moved by a two-helix finger domain of SecA that is inserted into the cytoplasmic opening of the SecY channel. Here we have used disulphide-bridge crosslinking to show that the loop at the tip of the two-helix finger of Escherichia coli SecA interacts with a polypeptide chain right at the entrance into the SecY pore. Mutagenesis demonstrates that a tyrosine in the loop is particularly important for translocation, but can be replaced by some other bulky, hydrophobic residues. We propose that the two-helix finger of SecA moves a polypeptide chain into the SecY channel with the tyrosine providing the major contact with the substrate, a mechanism analogous to that suggested for hexameric, protein-translocating ATPases.
Figures


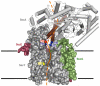
Comment in
-
Structural biology: Clamour for a kiss.Nature. 2008 Oct 16;455(7215):879-80. doi: 10.1038/455879a. Nature. 2008. PMID: 18923500 No abstract available.
References
-
- Rapoport TA. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature. 2007;450:663–669. - PubMed
-
- Brundage L, Hendrick JP, Schiebel E, Driessen AJM, Wickner W. The purified E. coli integral membrane protein SecY/E Is sufficient for reconstitution of SecA-dependent precursor proteintranslocation. Cell. 1990;62:649–657. - PubMed
-
- Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. - PubMed
-
- van den Berg B, et al. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

