Conformational transition of Sec machinery inferred from bacterial SecYE structures
- PMID: 18923527
- PMCID: PMC2590585
- DOI: 10.1038/nature07421
Conformational transition of Sec machinery inferred from bacterial SecYE structures
Abstract
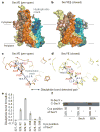
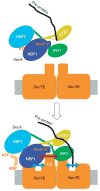
Over 30% of proteins are secreted across or integrated into membranes. Their newly synthesized forms contain either cleavable signal sequences or non-cleavable membrane anchor sequences, which direct them to the evolutionarily conserved Sec translocon (SecYEG in prokaryotes and Sec61, comprising alpha-, gamma- and beta-subunits, in eukaryotes). The translocon then functions as a protein-conducting channel. These processes of protein localization occur either at or after translation. In bacteria, the SecA ATPase drives post-translational translocation. The only high-resolution structure of a translocon available so far is that for SecYEbeta from the archaeon Methanococcus jannaschii, which lacks SecA. Here we present the 3.2-A-resolution crystal structure of the SecYE translocon from a SecA-containing organism, Thermus thermophilus. The structure, solved as a complex with an anti-SecY Fab fragment, revealed a 'pre-open' state of SecYE, in which several transmembrane helices are shifted, as compared to the previous SecYEbeta structure, to create a hydrophobic crack open to the cytoplasm. Fab and SecA bind to a common site at the tip of the cytoplasmic domain of SecY. Molecular dynamics and disulphide mapping analyses suggest that the pre-open state might represent a SecYE conformational transition that is inducible by SecA binding. Moreover, we identified a SecA-SecYE interface that comprises SecA residues originally buried inside the protein, indicating that both the channel and the motor components of the Sec machinery undergo cooperative conformational changes on formation of the functional complex.
Figures


Comment in
-
Structural biology: Clamour for a kiss.Nature. 2008 Oct 16;455(7215):879-80. doi: 10.1038/455879a. Nature. 2008. PMID: 18923500 No abstract available.
References
-
- Rapoport TA. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature. 2007;450:663–669. - PubMed
-
- Papanikou E, Karamanou S, Economou A. Bacterial protein secretion through the translocase nanomachine. Nat Rev Microbiol. 2007;5:839–851. - PubMed
-
- Vrontou E, Economou A. Structure and function of SecA, the preprotein translocase nanomotor. Biochim Biophys Acta. 2004;1694:67–80. - PubMed
-
- Van den Berg B, et al. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. - PubMed
-
- Mori H, Ito K. The Sec protein-translocation pathway. Trends Microbiol. 2001;9:494–500. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

