A nationwide survey of the quality of antimalarials in retail outlets in Tanzania
- PMID: 18923672
- PMCID: PMC2565501
- DOI: 10.1371/journal.pone.0003403
A nationwide survey of the quality of antimalarials in retail outlets in Tanzania
Abstract
Introduction: Retail pharmaceutical products are commonly used to treat fever and malaria in sub-Saharan African countries. Small scale studies have suggested that poor quality antimalarials are widespread throughout the region, but nationwide data are not available that could lead to generalizable conclusions about the extent to which poor quality drugs are available in African communities. This study aimed to assess the quality of antimalarials available from retail outlets across mainland Tanzania.
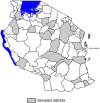
Methods and findings: We systematically purchased samples of oral antimalarial tablets from retail outlets across 21 districts in mainland Tanzania in 2005. A total of 1080 antimalarial formulations were collected including 679 antifol antimalarial samples (394 sulfadoxine/pyrimethamine and 285 sulfamethoxypyrazine/pyrimethamine), 260 amodiaquine samples, 63 quinine samples, and 51 artemisinin derivative samples. A systematic subsample of 304 products was assessed for quality by laboratory based analysis to determine the amount of the active ingredient and dissolution profile by following the published United States Pharmacopoeia (USP) monogram for the particular tablet being tested. Products for which a published analytical monogram did not exist were assessed on amount of active ingredient alone. Overall 38 or 12.2% of the samples were found to be of poor quality. Of the antifolate antimalarial drugs tested 13.4% were found to be of poor quality by dissolution and content analysis using high-performance liquid chromatography (HPLC). Nearly one quarter (23.8%) of quinine tablets did not comply within the tolerance limits of the dissolution and quantification analysis. Quality of amodiaquine drugs was relatively better but still unacceptable as 7.5% did not comply within the tolerance limits of the dissolution analysis. Formulations of the artemisinin derivatives all contained the stated amount of active ingredient when analysed using HPLC alone.
Conclusions: Substandard antimalarial formulations were widely available in Tanzania at the time of this study. No products were detected that did not contain any amount of the stated active ingredient. Quinine and sulfadoxine/pyrimethamine products were the most widely available and also the most likely to be of poor quality. Substandard products were identified in all parts of the country and were labeled as made by both domestic and international manufacturers. With the expansion of the retail pharmaceutical sector as a delivery channel for antimalarial formulations the need for regular nationwide monitoring of their quality will become increasingly important.
Conflict of interest statement
Figures
References
-
- Snow RW, Craig MH, Newton CRJC, Steketee RW. The Public Health Burden of Plasmodium falciparum Malaria in Africa:Deriving the Numbers. Disease Control Priorities Project Working Paper No. 11. Bethesda, Maryland: Fogarty International Center, National Institutes of Health; 2003.
-
- WHO . Counterfeit drugs: guidelines for the development of measures to combat counterfeit drugs. Geneva: WHO; 1999. pp. 1–60.
-
- WHO Counterfeit medicines. Fact sheet No 275. 2006. Revised February 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

