Evaluation of the sentinel immunized node for immune monitoring of cancer vaccines
- PMID: 18923873
- PMCID: PMC2997393
- DOI: 10.1245/s10434-008-0046-4
Evaluation of the sentinel immunized node for immune monitoring of cancer vaccines
Abstract
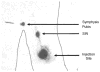
Background: We hypothesized that lymph nodes draining sites of cutaneous vaccination could be identified by sentinel node biopsy techniques, and that measuring T-cell response with lymphocytes obtained from these lymph nodes would provide a more sensitive measure of immunogenicity than would the same measurement made with peripheral blood lymphocytes (PBL).
Methods: ELISpot analysis was used to determine the magnitude of vaccine-specific T-cell response in the sentinel immunized nodes (SIN), random lymph nodes, and peripheral blood lymphocytes (PBL) obtained from patients enrolled in clinical trials of experimental melanoma vaccines.
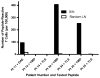
Results: The SIN biopsy was successful in 97% of cases and morbidity was very low. The T-cell response to vaccination was detected with greater sensitivity in the SIN (57%) than in PBL (39%), and evaluation of T-cell responses in the SIN and the PBL together yielded T-cell responses in 63% of patients. When the T-cell responses from a SIN and a random lymph node were compared in four patients, immune responses were detected to one of the vaccine peptides in three of these four patients. In all of those cases, responses were present in the SIN but absent from the random lymph node.
Conclusion: Measurements of T-cell responsiveness to cutaneous immunization are more frequently positive in the SIN than they are in the PBL, however evaluation of both the SIN and PBL permit a more sensitive measure of T-cell immunogenicity than use of either single source.
Figures

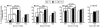

References
-
- Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. - PubMed
-
- Slingluff CL, Chianese-Bullock KA, Bullock TNJ, et al. Immunity to melanoma antigens: from self-tolerance to immunotherapy. Adv Immunol. 2006;90:243–95. - PubMed
-
- Marchand M, van Baren N, Weynants P, et al. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer. 1999;80:219–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

