Defective B-cell response to T-dependent immunization in lupus-prone mice
- PMID: 18924209
- PMCID: PMC2828936
- DOI: 10.1002/eji.200838417
Defective B-cell response to T-dependent immunization in lupus-prone mice
Abstract
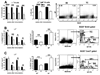
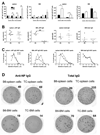
Lupus anti-nuclear Ab show the characteristics of Ag-driven T-cell-dependent (TD) humoral responses. If autoAg elicit the same response as exogenous Ag, lupus should enhance humoral responses to immunization. Blunted responses to various immunizations have, however, been reported in a significant portion of lupus patients. In this study, we show that lupus-prone C57BL/6.Sle1.Sle2.Sle3 (B6.TC) mice produce significantly less Ab in response to TD immunization than congenic controls, while producing significantly more total Ig. This blunted Ab response to TD Ag could be reconstituted with B6.TC B and CD4+ T cells. Multiple defects were found in the B6.TC response to 4-hydroxy-3-nitrophenylacetyl-keyhole limpet hemocyanin (NP-KLH) compared with total Ig, including a smaller percentage of B cells participating in the NP-response, a reduced entry into germinal centers, and highly defective production of NP-specific long-lived plasma cells (PC) in the bone marrow. B6.TC PC expressed reduced levels of FcgammaRIIb, which suggests that reduced apoptosis in resident PC prevents the establishment of newly formed NP-specific PC in bone marrow niches. Overall, these results show that lupus-prone mice responded differently to auto- and exogenous Ag and suggest that low FcgammaRIIb, hypergammaglobulinemia, and high autoAb production would be predictive of a poor response to immunization in lupus patients.
Figures






References
-
- Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat.Immunol. 2001;2:764–766. - PubMed
-
- Radic MZ, Weigert M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Ann.Rev.Immunol. 1994;12:487–520. - PubMed
-
- Craft J, Peng S, Fujii T, Okada M, Fatenejad S. Autoreactive T cells in murine lupus- Origins and roles in autoantibody production. Immunologic Research. 1999;19:245–257. - PubMed
-
- William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

