Notch1 is an effector of Akt and hypoxia in melanoma development
- PMID: 18924608
- PMCID: PMC2567838
- DOI: 10.1172/JCI36157
Notch1 is an effector of Akt and hypoxia in melanoma development
Abstract
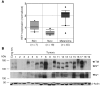
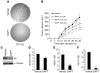
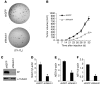
Melanomas are highly aggressive neoplasms resistant to most conventional therapies. These tumors result from the interaction of altered intracellular tumor suppressors and oncogenes with the microenvironment in which these changes occur. We previously demonstrated that physiologic skin hypoxia contributes to melanomagenesis in conjunction with Akt activation. Here we show that Notch1 signaling is elevated in human melanoma samples and cell lines and is required for Akt and hypoxia to transform melanocytes in vitro. Notch1 facilitated melanoma development in a xenograft model by maintaining cell proliferation and by protecting cells from stress-induced cell death. Hyperactivated PI3K/Akt signaling led to upregulation of Notch1 through NF-kappaB activity, while the low oxygen content normally found in skin increased mRNA and protein levels of Notch1 via stabilization of HIF-1alpha. Taken together, these findings demonstrate that Notch1 is a key effector of both Akt and hypoxia in melanoma development and identify the Notch signaling pathway as a potential therapeutic target in melanoma treatment.
Figures

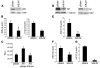


References
-
- Cannon-Albright L.A., Kamb A., Skolnick M. A review of inherited predisposition to melanoma. Semin. Oncol. 1996;23:667–672. - PubMed

