Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development
- PMID: 18924610
- PMCID: PMC2567839
- DOI: 10.1172/JCI36942
Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development
Abstract
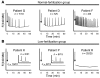
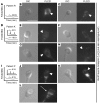
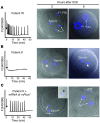
Egg activation, which is the first step in the initiation of embryo development, involves both completion of meiosis and progression into mitotic cycles. In mammals, the fertilizing sperm delivers the activating signal, which consists of oscillations in free cytosolic Ca(2+) concentration ([Ca(2+)](i)). Intracytoplasmic sperm injection (ICSI) is a technique that in vitro fertilization clinics use to treat a myriad of male factor infertility cases. Importantly, some patients who repeatedly fail ICSI also fail to induce egg activation and are, therefore, sterile. Here, we have found that sperm from patients who repeatedly failed ICSI were unable to induce [Ca(2+)](i) oscillations in mouse eggs. We have also shown that PLC, zeta 1 (PLCZ1), the sperm protein thought to induce [Ca(2+)](i) oscillations, was localized to the equatorial region of wild-type sperm heads but was undetectable in sperm from patients who had failed ICSI. The absence of PLCZ1 in these patients was further confirmed by Western blot, although genomic sequencing failed to reveal conclusive PLCZ1 mutations. Using mouse eggs, we reproduced the failure of sperm from these patients to induce egg activation and rescued it by injection of mouse Plcz1 mRNA. Together, our results indicate that the inability of human sperm to initiate [Ca(2+)](i) oscillations leads to failure of egg activation and sterility and that abnormal PLCZ1 expression underlies this functional defect.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

