The kinetics of CD4+Foxp3+ T cell accumulation during a human cutaneous antigen-specific memory response in vivo
- PMID: 18924611
- PMCID: PMC2556297
- DOI: 10.1172/JCI35834
The kinetics of CD4+Foxp3+ T cell accumulation during a human cutaneous antigen-specific memory response in vivo
Abstract
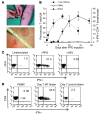
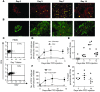
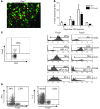
Naturally occurring CD4(+)CD25(hi)Foxp3(+) Tregs (nTregs) are highly proliferative in blood. However, the kinetics of their accumulation and proliferation during a localized antigen-specific T cell response is currently unknown. To explore this, we used a human experimental system whereby tuberculin purified protein derivative (PPD) was injected into the skin and the local T cell response analyzed over time. The numbers of both CD4(+)Foxp3(-) (memory) and CD4(+)Foxp3(+) (putative nTreg) T cells increased in parallel, with the 2 populations proliferating at the same relative rate. In contrast to CD4(+)Foxp3(-) T cell populations, skin CD4(+)Foxp3(+) T cells expressed typical Treg markers (i.e., they were CD25(hi), CD127(lo), CD27(+), and CD39(+)) and did not synthesize IL-2 or IFN-gamma after restimulation in vitro, indicating that they were not recently activated effector cells. To determine whether CD4(+)Foxp3(+) T cells in skin could be induced from memory CD4(+) T cells, we expanded skin-derived memory CD4(+) T cells in vitro and anergized them. These cells expressed high levels of CD25 and Foxp3 and suppressed the proliferation of skin-derived responder T cells to PPD challenge. Our data therefore demonstrate that memory and CD4(+) Treg populations are regulated in tandem during a secondary antigenic response. Furthermore, it is possible to isolate effector CD4(+) T cell populations from inflamed tissues and manipulate them to generate Tregs with the potential to suppress inflammatory responses.
Figures




References
-
- Shevach E.M. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2002;2:389–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

