Migration of antibody secreting cells towards CXCL12 depends on the isotype that forms the BCR
- PMID: 18925577
- PMCID: PMC2967815
- DOI: 10.1002/eji.200838456
Migration of antibody secreting cells towards CXCL12 depends on the isotype that forms the BCR
Abstract
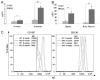
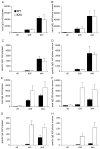
Truncation of the cytoplasmic tail of membrane-bound IgE in vivo results in lower serum IgE levels, decreased numbers of IgE-secreting plasma cells and the abrogation of specific secondary immune responses. Here we present mouse strain KN1 that expresses a chimeric epsilon-gamma1 BCR, consisting of the extracellular domains of the epsilon gene and the transmembrane and cytoplasmic domains of the gamma1 gene. Thus, differences in the IgE immune response of KN1 mice reflect the influence of the "gamma1-mediated signalling" of mIgE bearing B cells. KN1 mice show an increased serum IgE level, resulting from an elevated number of IgE-secreting cells. Although the primary IgE immune response in KN1 mice is inconspicuous, the secondary response is far more robust. Most strikingly, IgE-antibody secreting cells with "gamma1-signalling history" migrate more efficiently towards the chemokine CXCL12, which guides plasmablasts to plasma cell niches, than IgE-antibody secreting cells with WT "epsilon-signalling history". We conclude that IgE plasmablasts have an intrinsic, lower chance to contribute to the long-lived plasma cell pool than IgG1 plasmablasts.
Figures
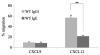



References
-
- Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu. Rev. Immunol. 2005;23:367–386. - PubMed
-
- McHeyzer-Williams MG. B cells as effectors. Curr. Opin. Immunol. 2003;15:354–361. - PubMed
-
- Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 2006;6:741–750. - PubMed
-
- Calame KL. Plasma cells: finding new light at the end of B cell development. Nat. Immunol. 2001;2:1103–1108. - PubMed
-
- Manz RA, Lohning M, Cassese G, Thiel A, Radbruch A. Survival of long-lived plasma cells is independent of antigen. Int. Immunol. 1998;10:1703–1711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

